Page 243 - pathology_services_handbook_5th_edition_2018
P. 243
• Prepare and issue annual report which include recommendation for corrective
action to the ministry of health.
• Upon receipt of the “REPORT OF REACTION TO BLOOD OR PLASMA” forms (PPDK
22-Pin. 1/80) by the Hospital Blood Bank, the reporting format for “Adverse
Transfusion Event (Borang X) will be issued to the ward / doctor concerned by
the Hospital Blood Bank. TRANSFUSION
• The “Adverse Transfusion Event” (Borang X) form will be returned in duplicate
to the Hospital Blood Bank and National Blood Centre within one week.
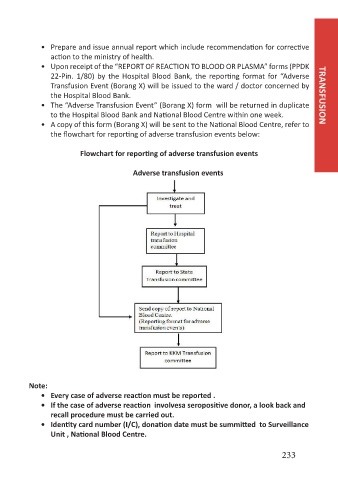
• A copy of this form (Borang X) will be sent to the National Blood Centre, refer to
the flowchart for reporting of adverse transfusion events below:
Flowchart for reporting of adverse transfusion events
Adverse transfusion events
Note:
• Every case of adverse reaction must be reported .
• If the case of adverse reaction involvesa seropositive donor, a look back and
recall procedure must be carried out.
• Identity card number (I/C), donation date must be summitted to Surveillance
Unit , National Blood Centre.
233