Page 100 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 100
YELLOW ZONE [YZ]
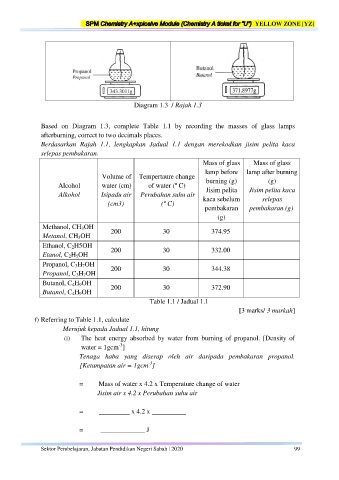
Diagram 1.3 / Rajah 1.3
Based on Diagram 1.3, complete Table 1.1 by recording the masses of glass lamps
afterburning, correct to two decimals places.
Berdasarkan Rajah 1.1, lengkapkan Jadual 1.1 dengan merekodkan jisim pelita kaca
selepas pembakaran.
Mass of glass Mass of glass
lamp before lamp after burning
Volume of Tempertaure change
(g)
Alcohol water (cm) of water (º C) burning (g) Jisim pelita kaca
Jisim pelita
Alkohol Isipadu air Perubahan suhu air kaca sebelum selepas
(cm3) (º C)
pembakaran pembakaran (g)
(g)
Methanol, CH 3OH 200 30 374.95
Metanol, CH 3OH
Ethanol, C 2H5OH
Etanol, C 2H 5OH 200 30 332.00
Propanol, C 3H 7OH 200 30 344.38
Propanol, C 3H 7OH
Butanol, C 4H 9OH 200 30 372.90
Butanol, C 4H 9OH
Table 1.1 / Jadual 1.1
[3 marks/ 3 markah]
f) Referring to Table 1.1, calculate
Merujuk kepada Jadual 1.1, hitung
(i) The heat energy absorbed by water from burning of propanol. [Density of
-3
water = 1gcm ]
Tenaga haba yang diserap oleh air daripada pembakaran propanol.
-3
[Ketumpatan air = 1gcm ]
= Mass of water x 4.2 x Temperature change of water
Jisim air x 4.2 x Perubahan suhu air
= _________ x 4.2 x __________
= _____________ J
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 99