Page 110 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 110
+
SPM Chemistry A xplosive Module (Chemistry A Ticket For “U” ) GREEN ZONE [GZ]
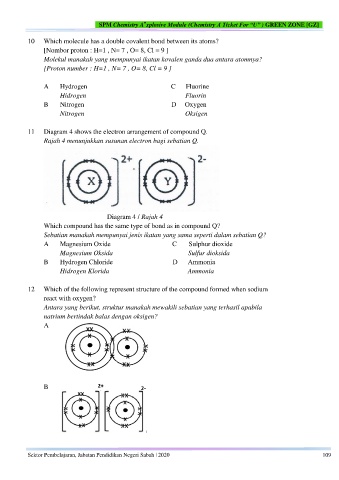
10 Which molecule has a double covalent bond between its atoms?
[Nombor proton : H=1 , N= 7 , O= 8, Cl = 9 ]
Molekul manakah yang mempunyai ikatan kovalen ganda dua antara atomnya?
[Proton number : H=1 , N= 7 , O= 8, Cl = 9 ]
A Hydrogen C Fluorine
Hidrogen Fluorin
B Nitrogen D Oxygen
Nitrogen Oksigen
11 Diagram 4 shows the electron arrangement of compound Q.
Rajah 4 menunjukkan susunan electron bagi sebatian Q.
Diagram 4 / Rajah 4
Which compound has the same type of bond as in compound Q?
Sebatian manakah mempunyai jenis ikatan yang sama seperti dalam sebatian Q?
A Magnesium Oxide C Sulphur dioxide
Magnesium Oksida Sulfur dioksida
B Hydrogen Chloride D Ammonia
Hidrogen Klorida Ammonia
12 Which of the following represent structure of the compound formed when sodium
react with oxygen?
Antara yang berikut, struktur manakah mewakili sebatian yang terhasil apabila
natrium bertindak balas dengan oksigen?
A
B
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 109