Page 140 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 140
+
SPM Chemistry A xplosive Module (Chemistry A Ticket For “U” ) GREEN ZONE [GZ]
3
(iii) Given 25.00 cm of HA acid is needed to reach the end point of neutralisation process in
-3
Diagram 4.1. Calculate the concentration of HA acid, in moldm .
3
Diberi 25.00cm asid HA diperlukan untuk mencapai titik akhir proses peneutralan di
-3
Rajah 4.1. Hitung kepekatan bagi asid HA, dalam moldm .
[2M]
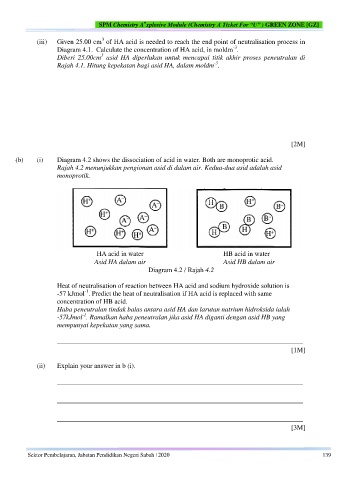
(b) (i) Diagram 4.2 shows the dissociation of acid in water. Both are monoprotic acid.
Rajah 4.2 menunjukkan pengionan asid di dalam air. Kedua-dua asid adalah asid
monoprotik.
HA acid in water HB acid in water
Asid HA dalam air Asid HB dalam air
Diagram 4.2 / Rajah 4.2
Heat of neutralisation of reaction between HA acid and sodium hydroxide solution is
-1
-57 kJmol . Predict the heat of neutralisation if HA acid is replaced with same
concentration of HB acid.
Haba peneutralan tindak balas antara asid HA dan larutan natrium hidroksida ialah
-1
-57kJmol . Ramalkan haba peneutralan jika asid HA diganti dengan asid HB yang
mempunyai kepekatan yang sama.
________________________________________________________________________
[1M]
(ii) Explain your answer in b (i).
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
[3M]
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 139