Page 205 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 205
+
SPM Chemistry A xplosive Module (Chemistry A Ticket For “U”) ANSWERS
4. (a) (i) P1 - Calcium nitrate/ Kalsium nitrat 2
P2 - Potassium carbonate // Sodium carbonate
Kalsium nitrat, Kalium carbonate/ Natrium carbonate
a: formula
a: any suitable answer
(ii) Ca(NO 3) 2 + K 2CO 3 CaCO 3 + 2KNO 3 2
P1 - correct reactant & product
P2 – balance chemical equation
(b) (i) P1 – Place near a moist blue litmus paper to the mouth of boiling tube 2
Dekatkan kertas litmus biru lembap kepada mulut tabung didih
P2 – Moist blue litmus paper turns red.
Kertas litmus biru lembap bertukar menjadi merah
(ii) zinc ion r: formula 1
(iii) Neutralisation 1
Peneutralan
(iv) Zinc carbonate // ZnCO 3 1
Zink karbonat
(v) Zinc carbonate // zinc hydroxide // zinc a: formula 1
zink karbonat// zink hidroksida // zink
10 marks
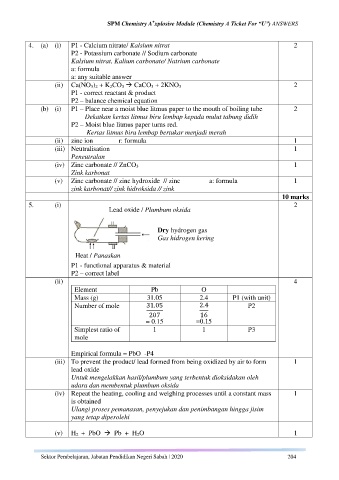
5. (i) 2
Lead oxide / Plumbum oksida
Dry hydrogen gas
Gas hidrogen kering
Heat / Panaskan
P1 - functional apparatus & material
P2 – correct label
(ii) 4
Element Pb O
Mass (g) 31.05 2.4 P1 (with unit)
Number of mole P2
= 0.15 =0.15
Simplest ratio of 1 1 P3
mole
Empirical formula = PbO -P4
(iii) To prevent the product/ lead formed from being oxidized by air to form 1
lead oxide
Untuk mengelakkan hasil/plumbum yang terbentuk dioksidakan oleh
udara dan membentuk plumbum oksida
(iv) Repeat the heating, cooling and weighing processes until a constant mass 1
is obtained
Ulangi proses pemanasan, penyejukan dan penimbangan hingga jisim
yang tetap diperolehi
(v) H 2 + PbO Pb + H 2O 1
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 204