Page 228 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 228
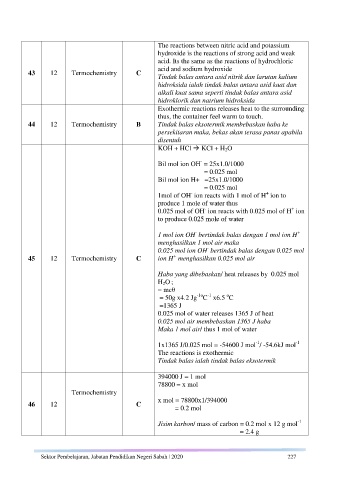
The reactions between nitric acid and potassium
hydroxide is the reactions of strong acid and weak
acid. Its the same as the reactions of hydrochloric
acid and sodium hydroxide
43 12 Termochemistry C
Tindak balas antara asid nitrik dan larutan kalium
hidroksida ialah tindak balas antara asid kuat dan
alkali kuat sama seperti tindak balas antara asid
hidroklorik dan natrium hidroksida
Exothermic reactions releases heat to the surrounding
thus, the container feel warm to touch.
44 12 Termochemistry B Tindak balas eksotermik membebaskan haba ke
persekitaran maka, bekas akan terasa panas apabila
disentuh
KOH + HCl KCl + H 2O
-
Bil mol ion OH = 25x1.0/1000
= 0.025 mol
Bil mol ion H+ =25x1.0/1000
= 0.025 mol
-
+
1mol of OH ion reacts with 1 mol of H ion to
produce 1 mole of water thus
-
+
0.025 mol of OH ion reacts with 0.025 mol of H ion
to produce 0.025 mole of water
-
+
1 mol ion OH bertindak balas dengan 1 mol ion H
menghasilkan 1 mol air maka
-
0.025 mol ion OH bertindak balas dengan 0.025 mol
+
45 12 Termochemistry C ion H menghasilkan 0.025 mol air
Haba yang dibebaskan/ heat releases by 0.025 mol
H 2O ;
= mcθ
o
-1o -1
= 50g x4.2 Jg C x6.5 C
=1365 J
0.025 mol of water releases 1365 J of heat
0.025 mol air membebaskan 1365 J haba
Maka 1 mol air/ thus 1 mol of water
-1
-1
1x1365 J/0.025 mol = -54600 J mol / -54.6kJ mol
The reactions is exothermic
Tindak balas ialah tindak balas eksotermik
394000 J = 1 mol
78800 = x mol
Termochemistry
x mol = 78800x1/394000
46 12 C
= 0.2 mol
-1
Jisim karbon/ mass of carbon = 0.2 mol x 12 g mol
= 2.4 g
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 227