Page 238 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 238
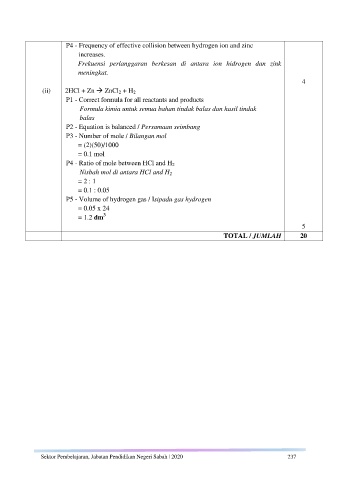
P4 - Frequency of effective collision between hydrogen ion and zinc
increases.
Frekuensi perlanggaran berkesan di antara ion hidrogen dan zink
meningkat.
4
(ii) 2HCl + Zn ZnCl 2 + H 2
P1 - Correct formula for all reactants and products
Formula kimia untuk semua bahan tindak balas dan hasil tindak
balas
P2 - Equation is balanced / Persamaan seimbang
P3 - Number of mole / Bilangan mol
= (2)(50)/1000
= 0.1 mol
P4 - Ratio of mole between HCl and H 2
Nisbah mol di antara HCl and H 2
= 2 : 1
= 0.1 : 0.05
P5 - Volume of hydrogen gas / Isipadu gas hydrogen
= 0.05 x 24
3
= 1.2 dm
5
TOTAL / JUMLAH 20
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 237