Page 32 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 32
+
SPM Chemistry A xplosive Module (Chemistry A Ticket For “U”) RED ZONE [RZ]
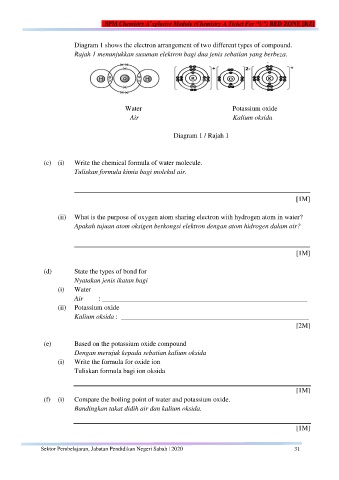
Diagram 1 shows the electron arrangement of two different types of compound.
Rajah 1 menunjukkan susunan elektron bagi dua jenis sebatian yang berbeza.
Water Potassium oxide
Air Kalium oksida
Diagram 1 / Rajah 1
(c) (i) Write the chemical formula of water molecule.
Tuliskan formula kimia bagi molekul air.
_____________________________________________________________________
[1M]
(ii) What is the purpose of oxygen atom sharing electron with hydrogen atom in water?
Apakah tujuan atom oksigen berkongsi elektron dengan atom hidrogen dalam air?
_____________________________________________________________________
[1M]
(d) State the types of bond for
Nyatakan jenis ikatan bagi
(i) Water
Air : ____________________________________________________________
(ii) Potassium oxide
Kalium oksida : _______________________________________________________
[2M]
(e) Based on the potassium oxide compound
Dengan merujuk kepada sebatian kalium oksida
(i) Write the formula for oxide ion
Tuliskan formula bagi ion oksida
[1M]
(f) (i) Compare the boiling point of water and potassium oxide.
Bandingkan takat didih air dan kalium oksida.
[1M]
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 31