Page 41 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 41
+
SPM Chemistry A xplosive Module (Chemistry A Ticket For “U”) RED ZONE [RZ]
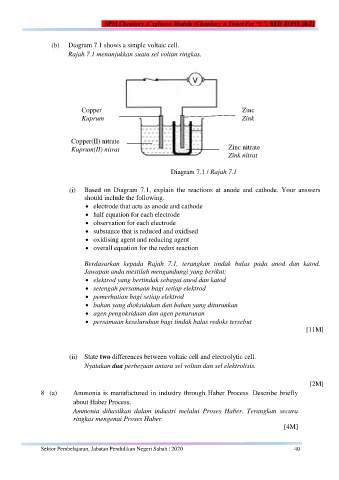
(b) Diagram 7.1 shows a simple voltaic cell.
Rajah 7.1 menunjukkan suatu sel voltan ringkas.
Copper Zinc
Kuprum Zink
Copper(II) nitrate Zinc nitrate
Kuprum(II) nitrat
Zink nitrat
Diagram 7.1 / Rajah 7.1
(i) Based on Diagram 7.1, explain the reactions at anode and cathode. Your answers
should include the following.
electrode that acts as anode and cathode
half equation for each electrode
observation for each electrode
substance that is reduced and oxidised
oxidising agent and reducing agent
overall equation for the redox reaction
Berdasarkan kepada Rajah 7.1, terangkan tindak balas pada anod dan katod.
Jawapan anda mestilah mengandungi yang berikut:
elektrod yang bertindak sebagai anod dan katod
setengah persamaan bagi setiap elektrod
pemerhatian bagi setiap elektrod
bahan yang dioksidakan dan bahan yang diturunkan
agen pengoksidaan dan agen penurunan
persamaan keseluruhan bagi tindak balas redoks tersebut
[11M]
(ii) State two differences between voltaic cell and electrolytic cell.
Nyatakan dua perbezaan antara sel voltan dan sel elektrolisis.
[2M]
8 (a) Ammonia is manufactured in industry through Haber Process. Describe briefly
about Haber Process.
Ammonia dihasilkan dalam industri melalui Proses Haber. Terangkan secara
ringkas mengenai Proses Haber.
[4M]
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 40