Page 2526 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2526
Chapter 155 Hematologic Manifestations of Malignancy 2249
contribute to tumor progression. Platelets derived from cancer white blood cell counts, low body mass index/BSA, advanced disease,
patients are in an activated state, with increased surface P-selectin and the use of myelosuppressive chemotherapy agents. General treat-
expression and elevated serum levels of platelet factor IV and ment principles for neutropenic fever include the consideration of
β-thromboglobulin. Activated platelets may contribute to the throm- prophylactic antibiotics or myeloid growth factor support, such as
bophilic state in cancer patients, and also interact with leukocytes, granulocyte colony-stimulating factor (G-CSF). Consideration
endothelial cells, and tumor cells, all of which may contribute to the should be given to the elevated risk of FN associated with specific
early stages of tumor cell dissemination. A number of platelet recep- chemotherapeutic agents, including situations where dose-dense or
tors may contribute to the platelet support of metastasis, including intense chemotherapy regimens provide survival benefit. Depending
glycoprotein (GP) IIb/IIIa, adenosine diphosphate (ADP) receptors, on the regimen and disease, prophylactic G-CSF should be consid-
P-selectin and thrombin receptors, and others, with likely multiple ered, particularly when there is a greater than 20% overall risk of FN;
mechanisms underlying the platelet effects on malignancy. Platelets once a patient develops FN, G-CSF is typically reserved for situations
stabilize otherwise short-lived tumor cells in the circulation. Platelet– where they are not responding to appropriate treatment and develop
tumor cell interaction enhances tumor cell adhesion to the vessel wall, life-threatening conditions.
and appears to alter vessel wall permeability, and in this way may Several tumors express G-CSF receptors; there is a theoretic
facilitate tumor cell invasion. Platelets protect tumor cells from concern that exogenous G-CSF may increase proliferation of these
immune surveillance and destruction, in part by shielding tumor cells tumors. In addition, the use of myeloid growth factors during che-
from natural killer cells, although other mechanisms are likely. motherapy for solid tumors has been theorized to increase the risk of
Platelets provide nutrient support (growth factors) and release pro- subsequent myeloid malignancy by acting as a survival signal to
angiogenic factors. Platelets contain numerous growth factors, coagu- hematopoietic progenitor cells damaged by chemotherapy. A meta-
lation factors and adhesive molecules, chemokines, and bioactive analysis of 25 randomized clinical trials found that there was an
lipids that may enhance metastatic efficiency. Platelets are enriched almost twofold increase in acute myeloid leukemia in those patients
in angiogenic growth factors such as basic fibroblast growth factor assigned to receive chemotherapy with growth factor support, com-
and VEGF, as well as other mediators, and may regulate angiogenesis. pared with those who did not receive growth factor support, although
Selective P-selectin and thrombin receptor activation on platelets may the absolute rates of secondary leukemia were low in each group.
release α-granules enriched in either VEGF or endostatin. Platelet- An important consideration during chemotherapy is to recognize
derived transforming growth factor-β and direct platelet–tumor cell that benign neutropenia related to certain ethnicities, particularly in
interactions promote epithelial mesenchymal transformation and those of African and Middle Eastern descent, may be a contributing
tumor metastases. factor and does not require dose-adjustment of chemotherapy.
LEUKOCYTES BONE MARROW METASTASES
Neutropenia Multiple lineage cytopenia in association with cancer warrants con-
sideration of bone marrow metastasis (Fig. 155.1). Metastatic carci-
Neutropenia is common during treatment with chemotherapy, noma can involve bone marrow and may lead to subsequent marrow
particularly with antimetabolite use. Other etiologies of neutropenia fibrosis and failure. Bone marrow metastases occur in fewer than 10%
in cancer patients include infiltration of the bone marrow space of patients with metastatic disease, and are more common in patients
and occasionally, radiation therapy, particularly when sites of active with lung, breast, or prostate carcinoma. Patients with solid tumors
hematopoiesis are included in the radiation field, such as with the who have bone marrow involvement are more likely to experience
pelvis or spine. In addition, particularly in the course of treat- cytopenias from chemotherapy, and typically carry a worse prognosis.
ment, cancer patients may experience viral or antibiotic-mediated For example, patients with extensive small cell lung cancer with bone
neutropenia. marrow metastases have significantly shorter time to progression and
Patients who develop neutropenia during chemotherapy are at risk significantly shorter survival time than other patients with extensive
for neutropenic fever. A number of comorbid conditions are predic- disease. The precise mechanism for poor outcomes is unknown, but
tive of the development of neutropenic fever, including older age, may relate to greater overall tumor burden, as well as to specific
poor performance status, comorbid medical conditions, low baseline biologic features that allow the tumor to grow in the bone marrow
A B C
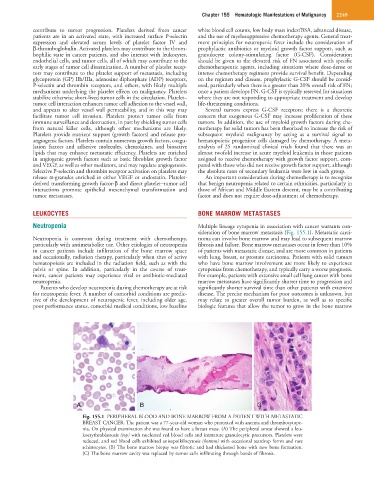
Fig. 155.1 PERIPHERAL BLOOD AND BONE MARROW FROM A PATIENT WITH METASTATIC
BREAST CANCER. The patient was a 77-year-old woman who presented with anemia and thrombocytope-
nia. On physical examination she was found to have a breast mass. (A) The peripheral smear showed a leu-
koerythroblastosis (top) with nucleated red blood cells and immature granulocytic precursors. Platelets were
reduced, and red blood cells exhibited anisopoikilocytosis (bottom) with occasional teardrop forms and rare
schistocytes. (B) The bone marrow biopsy was fibrotic and had thickened bone with new bone formation.
(C) The bone marrow cavity was replaced by tumor cells infiltrating through bands of fibrosis.