Page 677 - Williams Hematology ( PDFDrive )
P. 677
652 Part VI: The Erythrocyte Chapter 44: Anemia Resulting From Other Nutritional Deficiencies 653
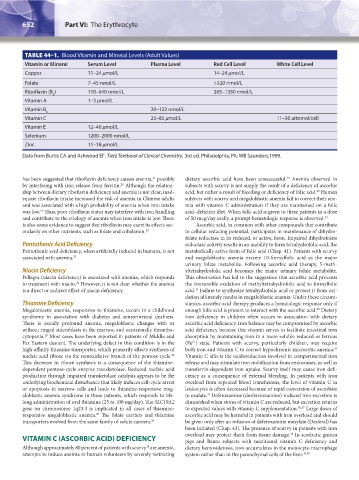
TABLE 44–1. Blood Vitamin and Mineral Levels (Adult Values)
Vitamin or Mineral Serum Level Plasma Level Red Cell Level White Cell Level
Copper 11–24 μmol/L 14–24 μmol/L
Folate 7–45 nmol/L >320 nmol/L
Riboflavin (B ) 110–640 nmol/L 265–1350 nmol/L
2
Vitamin A 1–3 μmol/L
Vitamin B 20–122 nmol/L
6
Vitamin C 25–85 μmol/L 11–30 attomol/cell
Vitamin E 12–40 μmol/L
Selenium 1200–2000 nmol/L
Zinc 11–18 μmol/L
Data from Burtis CA and Ashwood EF: Tietz Textbook of Clinical Chemistry, 3rd ed. Philadelphia, PA: WB Saunders,1999.
21
30
has been suggested that riboflavin deficiency causes anemia, possibly dietary ascorbic acid have been unsuccessful. Anemia observed in
20
by interfering with iron release from ferritin. Although the relation- subjects with scurvy is not simply the result of a deficiency of ascorbic
ship between dietary riboflavin deficiency and anemia is not clear, inad- acid, but rather a result of bleeding or deficiency of folic acid. Human
29
equate riboflavin intake increased the risk of anemia in Chinese adults subjects with scurvy and megaloblastic anemia fail to correct their ane-
and was associated with a high probability of anemia when iron intake mia with vitamin C administration if they are maintained on a folic
16
was low. Thus, poor riboflavin status may interfere with iron handling acid–deficient diet. When folic acid is given to these patients in a dose
and contribute to the etiology of anemia when iron intake is low. There of 50 mcg/day orally, a prompt hematologic response is observed. 31
is also some evidence to suggest that riboflavin may exert its effects sec- Ascorbic acid, in common with other compounds that contribute
ondarily on other nutrients, such as folate and cobalamin. 22 to cellular reducing potential, participates in maintenance of dihydro-
folate reductase in its reduced, or active, form. Impaired dihydrofolate
Pantothenic Acid Deficiency reductase activity results in an inability to form tetrahydrofolic acid, the
Pantothenic acid deficiency, when artificially induced in humans, is not metabolically active form of folic acid (Chap. 41). Patients with scurvy
associated with anemia. 23 and megaloblastic anemia excrete 10-formylfolic acid as the major
urinary folate metabolite. Following ascorbic acid therapy, 5-meth-
Niacin Deficiency yltetrahydrofolic acid becomes the major urinary folate metabolite.
Pellagra (niacin deficiency) is associated with anemia, which responds This observation has led to the suggestion that ascorbic acid prevents
to treatment with niacin. However, it is not clear whether the anemia the irreversible oxidation of methyltetrahydrofolic acid to formylfolic
24
is a direct or indirect effect of niacin deficiency. acid. Failure to synthesize tetrahydrofolic acid or protect it from oxi-
32
dation ultimately results in megaloblastic anemia. Under these circum-
Thiamine Deficiency stances, ascorbic acid therapy produces a hematologic response only if
Megaloblastic anemia, responsive to thiamine, occurs in a childhood enough folic acid is present to interact with the ascorbic acid. Dietary
33
syndrome in association with diabetes and sensorineural deafness. iron deficiency in children often occurs in association with dietary
There is usually profound anemia, megaloblastic changes with or ascorbic acid deficiency. Iron balance may be compromised by ascorbic
without ringed sideroblasts in the marrow, and occasionally thrombo- acid deficiency because this vitamin serves to facilitate intestinal iron
cytopenia. Most cases have been reported in patients of Middle and absorption by maintaining iron in a more soluble reduced or ferrous
25
Far Eastern descent. The underlying defect in this condition is in the (Fe ) state. Patients with scurvy, particularly children, may require
2+
34
high-affinity thiamine transporter, which primarily affects synthesis of both iron and vitamin C to correct hypochromic microcytic anemia.
nucleic acid ribose via the nonoxidative branch of the pentose cycle. Vitamin C affects the oxidoreduction involved in compartmental iron
26
This decrease in ribose synthesis is a consequence of the thiamine- release and may stimulate iron mobilization from endosomes, as well as
dependent pentose-cycle enzyme transketolase. Reduced nucleic acid transferrin-dependent iron uptake. Scurvy itself may cause iron defi-
production through impaired transketolase catalysis appears to be the ciency as a consequence of external bleeding. In patients with iron
underlying biochemical disturbance that likely induces cell-cycle arrest overload from repeated blood transfusions, the level of vitamin C in
or apoptosis in marrow cells and leads to thiamine-responsive meg- leukocytes is often decreased because of rapid conversion of ascorbate
aloblastic anemia syndrome in these patients, which responds to life- to oxalate. Deferoxamine (desferrioxamine)-induced iron excretion is
35
long administration of oral thiamine (25 to 100 mg/day). The SLC19A2 diminished when stores of vitamin C are reduced, but excretion returns
gene on chromosome 1q23.3 is implicated in all cases of thiamine- to expected values with vitamin C supplementation. 36,37 Large doses of
27
responsive megaloblastic anemia. The folate carriers and thiamine ascorbic acid may be harmful in patients with iron overload and should
transporters evolved from the same family of solute carriers. 28 be given only after an infusion of deferoxamine mesylate (Desferal) has
been initiated (Chap. 43). The presence of scurvy in patients with iron
38
VITAMIN C (ASCORBIC ACID) DEFICIENCY overload may protect them from tissue damage. In scorbutic guinea
pigs and Bantu subjects with nutritional vitamin C deficiency and
Although approximately 80 percent of patients with scurvy are anemic, dietary hemosiderosis, iron accumulates in the monocyte-macrophage
29
attempts to induce anemia in human volunteers by severely restricting system rather than in the parenchymal cells of the liver. 39,40
Kaushansky_chapter 44_p0651-0656.indd 652 9/17/15 6:30 PM