Page 1 - Scapho-PullUp3-5 years of proven efficacy FA
P. 1
Take early control in psoriatic disease with a
Complete Treatment Approach*
5 years of proven efficacy and safety with
Secukinumab (Scapho )
™
Secukinumab (Scapho ) delivers long-lasting skin clearance 2
™
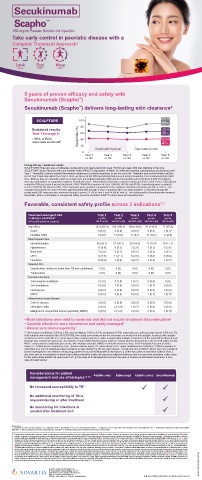
SCULPTURE 100 PASI 75
89%
80
Sustained results PASI 80
Year 1 through 5: 60 66%
-100% of PASI Responder (%) 40 PASI 100
responses sustained 2 41%
20
Double-blind period Open-label period
0
Year 1 Year 2 Year 3 Year 4 Year 5
n+182 n+152 n+139 n+132 n+122
Strong efficacy / Sustained results:
SCULPTURE Extension was a multicenter, double-blind and open-label (from week 156 through week 260) trial. Methods In the core
SCULPTURE study, Psoriasis Area and Severity Index (PASI) 75 responders at Week 12 continued receiving subcutaneous secukinumab until
Year 1. Thereafter, patients entered the extension phase and continued treatment as per the core trial. Treatment was double-blinded until the
end of Year 3 and open-label from Year 4. Here, we focus on the 300 mg fixed-interval (every 4 weeks) treatment, the recommended per label
dose. Efficacy data are primarily reported as observed, but multiple imputation (MI) and last observation carried forward (LOCF) techniques
were also undertaken as supportive analyses. Results At Year 1, 168 patients entered the extension study and at the end of Year 5, 126 patients
completed 300 mg (every 4 weeks) treatment. PASI 75/90/100 responses at Year 1 (88.9%, 68.5% and 43.8%, respectively) were sustained
to Year 5 (88.5%, 66.4% and 41%). PASI responses were consistent regardless of the analysis undertaken (as observed, MI, or LOCF). The
average improvement in mean PASI was approximately 90% through 5 years compared with core study baseline. DLQI (dermatology life
quality index) 0/1 response also sustained through 5 years (72.7% at Year 1 and 65.5% at Year 5). The safety profile of secukinumab remained
favourable, with no cumulative or unexpected safety concerns identified. PASI=Psoriasis Area and Severity Index
Favorable, consistent safety profile across 3 indications 1,2
Treatment-emergent AEs Year 1 Year 2 Year 3 Year 4 Year 5
in plaque psoriasis* (n=168) (n=168) (n=157) (n=142) (n=134)
300-mg (FI) extension subgroup 168 PY n (IR) 162.8 PY n (IR) 148.8 PY n (IR) 136.5 PY n (IR) 142 PY n (IR)
Any AE(s) 131 (204.6) 126 (166.3) 109 (139.2) 91 (118.5) 77 (87.2)
Death 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) †
Nonfatal SAEs 14 (8.8 11 (6.9) 13 (9.1) 13 (10.1) 11 (8.0)
Most frequent AEs
Nasopharyngitis 30 (20.1) 27 (18.1) 25 (18.8) 17 (13.5) 15 (11.1)
Hypertension 11 (6.8) 8 (5.1) 3 (2.0) 7 (5.3) 5 (3.6)
Back pain 7 (4.3) 9 (5.7) 9 (6.2) 3 (2.2) 3 (2.1)
URTI 12 (7.5) 11 (7.1) 5 (3.5) 5 (3.8) 5 (3.6)
Headache 10 (6.2) 7 (4.4) 4 (2.7) 3 (2.2) 1 (0.7)
Selected AEs
Opportunistic infections (other than TB and candidiasis) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0)
Tuberculosis 0 (0) 0 (0) 0 (0) 0 (0) 0 (0)
Candida infections
Vulvovaginal candidiasis 3 (1.8) 3 (1.9) 1 (0.7) 0 (0.0) 0 (0)
Oral candidiasis 0 (0.0) 1 (0.6) 0 (0.0) 1 (0.7) 0 (0.0)
Neutropenia 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0)
MACE 0 (0.0) 0 (0.0) 0 (0.0) 1 (0.7) 1 (0.7) †
Inflammatory bowel disease
Crohn’s disease 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0)
Ulcerative colitis 0 (0.0) 2 (1.2) ‡ 1 (0.7) 0 (0.0) 0 (0.0)
Malignant or unspecified tumors (excluding NMSC) 0 (0.0) 2 (1.2) § 0 (0.0) 0 (0.0) 1 (0.7) ||
• Most infections were mild to moderate and did not require treatment discontinuation 2
• Candida infections were uncommon and easily managed 2
• Almost zero immunogenicity 1
** Vulvovaginal candidiasis: 0.0% to 1.9%; oral candidiasis: 0.0% to 0.7%; neutropenia 0.0%; major adverse cardiovascular events: 0.0% to 0.7%
Only subjects who completed the SCULPTURE core study and continued into the extension are included in this analysis. A subject with multiple
occurrences of the same AE in a 1-year interval was counted only once, while a subject with multiple occurrences of the same AE in different year
intervals was counted for each year. AE=adverse event; CNS=central nervous system; FI=fixed interval; IR=incidence rate per 100 subject years;
MACE=major adverse cardiovascular events; MS=multiple sclerosis; NMSC=nonmelanoma skin cancer; PASI=Psoriasis Area and Severity
Index; PY=patient years of exposure; SAE=serious adverse event; TB=tuberculosis; URTI=upper respiratory tract infection * Patient exposure is
calculated as a sum of individual subject durations in days divided by 365 for each interval. † Death was due to MACE, which was not considered
by the investigators to be related to study drug; patient had ≥2 preexisting MACE risk factors. ‡ Of the two cases of ulcerative colitis in Year 2,
one case was an exacerbation of previously existing ulcerative colitis; the exposure-adjusted incidence rate for new-onset ulcerative colitis cases
for the entire study duration (5 years) was 0.27. § One case of cholangiocarcinoma and one case of invasive ductal breast carcinoma. II One
case of breast cancer.
Considerations for patient
management and use of biologics 1,4,5,6 Adalimumab Etanercept Ustekinumab Secukinumab
No increased susceptibility to TB*
No additional monitoring of TB is
required during or after treatment
No monitoring for infections is
needed after treatment end
References:
1. Secukinumab Core Data Sheet; version 2.4; 23 September 2019 2. Bissonnette R, et al. J Eur Acad Dermatol Venereol. 3. Langley RG, et al. N Engl J Med. 2014;371(4):326-338. 2018;32(9):1507-1514 4. Adalimumab Summary of Product Characteristics.
May 2019. 5. Etanercept Summary of Product Characteristics. April 2019. 6. Ustekinumab Summary of Product Characteristics. February 2020
Presentation: Secukinumab. Powder for solution for subcutaneous injection containing 150 mg of secukinumab. Indications: Plaque psoriasis: Treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. Psoriatic
arthritis: Treatment of active psoriatic arthritis in adult patients, alone or in combination with methotrexate (MTX), when the response to previous disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate. Ankylosing spondylitis: Treatment
of active ankylosing spondylitis in adults who have responded inadequately to conventional therapy. Dosage and administration: Plaque psoriasis: The recommended dose is 300 mg by subcutaneous injection with initial dosing at weeks 0, 1, 2, 3, and 4
followed by monthly maintenance dosing. Each 300 mg dose is given as two subcutaneous injections of 150 mg. Psoriatic arthritis: The recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly
maintenance dosing. Based on clinical response, the dose can be increased to 300 mg. ; For patients who are anti-TNF-alpha inadequate responders or patients with concomitant moderate to severe plaque psoriasis, the recommended dose is 300 mg by
subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing. Each 300 mg dose is given as two subcutaneous injections of 150 mg. Ankylosing spondylitis: The recommended dose is 150 mg by subcutaneous
injection with initial dosing at Weeks 0, 1, 2, 3, and 4 followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg. Each 300 mg dose is given as two subcutaneous injections of 150 mg.; Contraindications:
Patients who have/had severe hypersensitivity reactions reaction to the active substance or to any of the excipients. Warnings and precautions: Infections: Caution in patients with chronic or history of recurrent infection. If a patient develops a serious
infection, the patient should be closely monitored and secukinumab should not be administered until the infection resolves. Anti-tuberculosis therapy should be considered prior to initiation of treatment in patients with latent tuberculosis. Secukinumab should
not be given to patients with active tuberculosis. Inflammatory bowel disease: Patients with active inflammatory bowel disease (e.g. Crohn’s disease and ulcerative colitis) treated with Scapho should be followed closely. Hypersensitivity reactions: Rare
cases of anaphylactic reactions have been observed during clinical trials. Administration of secukinumab should be discontinued immediately and appropriate therapy initiated if an anaphylactic or other serious allergic reaction occurs. Vaccinations: Live
vaccines should not be given concurrently. Pregnancy, lactation, females and males of reproductive potential Pregnancy: Treatment should be used during pregnancy only if the benefits clearly outweigh the potential risks. Lactation: Caution should
be exercised when administered to a woman who is breast-feeding. Adverse drug reactions: Very common (>_10%): Upper respiratory tract infections (nasopharyngitis, upper respiratory tract infection, rhinitis, pharyngitis, sinusitis, tonsillitis).Common (1 to
<10%): Oral herpes, diarrhea, urticaria, rhinorrhea.Uncommon (0.1 to <1%): Oral candidiasis, neutropenia, tinea pedis, conjunctivitis.Frequency not known: Mucosal and cutaneous candidiasis Interactions: Live vaccines should not be given concurrently. In
a study in subjects with plaque psoriasis, no interaction was observed between secukinumab and midazolam (CYP 3A4 substrate).
Novartis Healthcare Philippines, Inc. I-SCAPH-02032022-PH2103047786
5F Ayala North Exchange Building, Tower 1
Ayala Avenue corner Salcedo & Amorsolo Sts,
Brgy. San Lorenzo, Makati City 1223
Tel. Nos.: +632-7368-7777 • Fax No.: +632-8816-4191 Full prescribing information available upon request.