Page 16 - ACE YR IGCSE A TOP APPROACH TO CHEM
P. 16
Part 2: Structured Questions
Answer all questions.
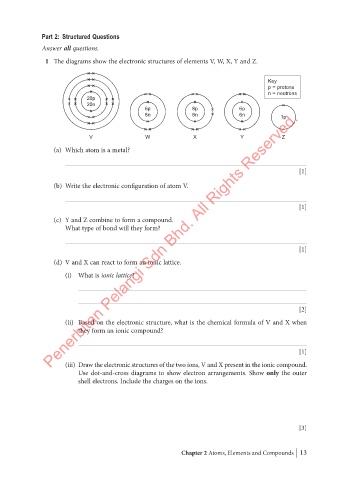
1 The diagrams show the electronic structures of elements V, W, X, Y and Z.
Key
p = protons
n = neutrons
20p
20n
6p 8p 6p
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
8n 8n 6n 1p
V W X Y Z
(a) Which atom is a metal?
[1]
(b) Write the electronic configuration of atom V.
[1]
(c) Y and Z combine to form a compound.
What type of bond will they form?
[1]
(d) V and X can react to form an ionic lattice.
(i) What is ionic lattice?
[2]
(ii) Based on the electronic structure, what is the chemical formula of V and X when
they form an ionic compound?
[1]
(iii) Draw the electronic structures of the two ions, V and X present in the ionic compound.
Use dot-and-cross diagrams to show electron arrangements. Show only the outer
shell electrons. Include the charges on the ions.
[3]
Chapter 2 Atoms, Elements and Compounds 13
Chapter 2.indd 13 3/4/22 11:59 AM