Page 10 - chemi
P. 10
+WHY USE CHROMIUM IN ELECTROPLATING
-has a high melting point, stable crystalline structure, and moderate thermal
expansion
-produce a shiny, hard surface
-does not corrode
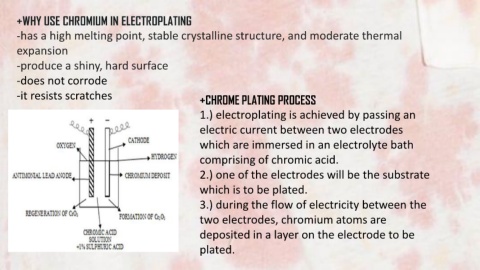
-it resists scratches +CHROME PLATING PROCESS
1.) electroplating is achieved by passing an
electric current between two electrodes
which are immersed in an electrolyte bath
comprising of chromic acid.
2.) one of the electrodes will be the substrate
which is to be plated.
3.) during the flow of electricity between the
two electrodes, chromium atoms are
deposited in a layer on the electrode to be
plated.