Page 29 - EOD Final
P. 29
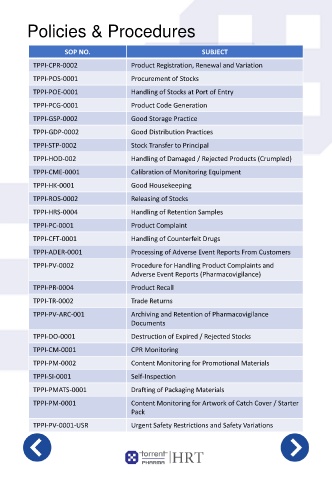
Policies & Procedures
SOP NO. SUBJECT
TPPI-CPR-0002 Product Registration, Renewal and Variation
TPPI-POS-0001 Procurement of Stocks
TPPI-POE-0001 Handling of Stocks at Port of Entry
TPPI-PCG-0001 Product Code Generation
TPPI-GSP-0002 Good Storage Practice
TPPI-GDP-0002 Good Distribution Practices
TPPI-STP-0002 Stock Transfer to Principal
TPPI-HOD-002 Handling of Damaged / Rejected Products (Crumpled)
TPPI-CME-0001 Calibration of Monitoring Equipment
TPPI-HK-0001 Good Housekeeping
TPPI-ROS-0002 Releasing of Stocks
TPPI-HRS-0004 Handling of Retention Samples
TPPI-PC-0001 Product Complaint
TPPI-CFT-0001 Handling of Counterfeit Drugs
TPPI-ADER-0001 Processing of Adverse Event Reports From Customers
TPPI-PV-0002 Procedure for Handling Product Complaints and
Adverse Event Reports (Pharmacovigilance)
TPPI-PR-0004 Product Recall
TPPI-TR-0002 Trade Returns
TPPI-PV-ARC-001 Archiving and Retention of Pharmacovigilance
Documents
TPPI-DO-0001 Destruction of Expired / Rejected Stocks
TPPI-CM-0001 CPR Monitoring
TPPI-PM-0002 Content Monitoring for Promotional Materials
TPPI-SI-0001 Self-Inspection
TPPI-PMATS-0001 Drafting of Packaging Materials
TPPI-PM-0001 Content Monitoring for Artwork of Catch Cover / Starter
Pack
TPPI-PV-0001-USR Urgent Safety Restrictions and Safety Variations