Page 315 - Ultimate Visual Dictionary (DK)
P. 315
CHEMICAL REACTIONS
Potassium iodide
solution added to
lead nitrate
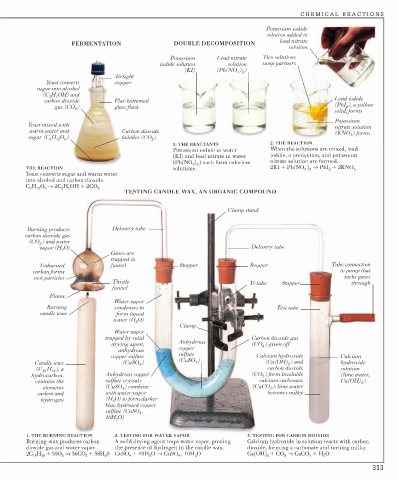
FERMENTATION DOUBLE DECOMPOSITION
solution
Potassium Lead nitrate Two solutions
iodide solution solution swap partners
) )
(KI) (Pb(NO 3 2
Airtight
Yeast converts stopper
sugar into alcohol
(C H OH) and
2
5
carbon dioxide Flat-bottomed Lead iodide
gas (CO ) glass flask (PbI 2 ), a yellow
2
solid, forms
Potassium
Yeast mixed with nitrate solution
warm water and Carbon dioxide (KNO 3 ) forms
sugar (C H O ) bubbles (CO 2 )
6
12 6
1. THE REACTANTS 2. THE REACTION
Potassium iodide in water When the solutions are mixed, lead
(KI) and lead nitrate in water iodide, a precipitate, and potassium
(Pb(NO ) ) each form colorless nitrate solution are formed.
3 2
THE REACTION solutions. 2KI + Pb(NO ) → PbI + 2KNO 3
2
3 2
Yeast converts sugar and warm water
into alcohol and carbon dioxide.
C H O → 2C H OH + 2CO 2
2
5
6
12 6
TESTING CANDLE WAX, AN ORGANIC COMPOUND
Clamp stand
Burning produces Delivery tube
carbon dioxide gas
(CO 2 ) and water
vapor (H 2 O) Delivery tube
Gases are
trapped in
Unburned funnel Stopper Stopper Tube connection
carbon forms to pump that
soot particles sucks gases
Thistle U-tube Stopper through
funnel
Flame
Water vapor
Burning condenses to Test tube
candle wax form liquid
water (H 2 O)
Clamp
Water vapor
trapped by solid Carbon dioxide gas
drying agent, Anhydrous (CO 2 ) given off
anhydrous copper
copper sulfate sulfate Calcium hydroxide Calcium
4
Candle wax (CuSO 4 ) (CuSO ) (Ca(OH) ) and hydroxide
2
(C 18 H 38 ), a carbon dioxide solution
hydrocarbon, Anhydrous copper (CO ) form insoluble (lime water,
2
contains the sulfate crystals calcium carbonate Ca(OH) 2 )
elements (CuSO ) combine (CaCO ): lime water
3
4
carbon and with water vapor becomes milky
hydrogen (H O) to form darker
2
blue hydrated copper
sulfate (CuSO 4 .
10H 2 O)
1. THE BURNING REACTION 2. TESTING FOR WATER VAPOR 3. TESTING FOR CARBON DIOXIDE
Burning wax produces carbon A solid drying agent traps water vapor, proving Calcium hydroxide in solution reacts with carbon
dioxide gas and water vapor. the presence of hydrogen in the candle wax. dioxide, forming a carbonate and turning milky.
2C 18 H 38 + 55O 2 → 36CO 2 + 38H 2 0 CuSO 4 + 10H 2 O → CuSO 4 . 10H 2 O Ca(OH) 2 + CO 2 → CaCO 3 + H 2 O
313