Page 20 - Nilam_Publication_module_Chemistry_Form.pdf
P. 20
MODULE • Chemistry Form 4
EXERCISE / LATIHAN
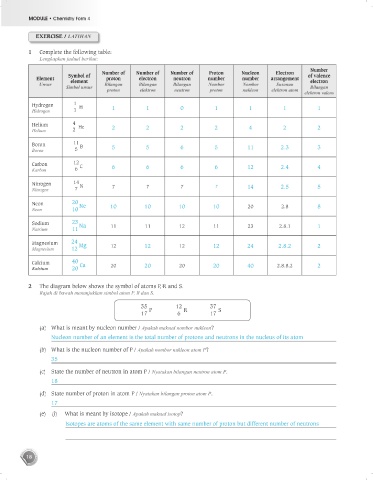
1 Complete the following table:
Lengkapkan jadual berikut:
Number
Number of Number of Number of Proton Nucleon Electron
Symbol of of valence
Element element proton electron neutron number number arrangement electron
Unsur Bilangan Bilangan Bilangan Nombor Nombor Susunan
Simbol unsur Bilangan
proton elektron neutron proton nukleon elektron atom
elektron valens
Hydrogen 1 H 1 1 0 1 1 1 1
Hidrogen 1
Helium 4 He 2 2 2 2 4 2 2
Helium 2
Boron 11 B 5 5 6 5 11 2.3 3
Boron 5
Carbon 12 C 6 6 6 6 12 2.4 4
Karbon 6
Nitrogen 14 N 7 7 7 7 14 2.5 5
Nitrogen 7
Neon 20 Ne 10 10 10 10 20 2.8 8
Neon 10
Sodium 23 Na 11 11 12 11 23 2.8.1 1
Natrium 11
Magnesium 24 Mg 12 12 12 12 24 2.8.2 2
Magnesium 12
Calcium 40 Ca 20 20 20 20 40 2.8.8.2 2
Kalsium 20
2 The diagram below shows the symbol of atoms P, R and S.
Rajah di bawah menunjukkan simbol atom P, R dan S.
35 12 37
17 P 6 R 17 S
(a) What is meant by nucleon number / Apakah maksud nombor nukleon?
Nucleon number of an element is the total number of protons and neutrons in the nucleus of its atom
(b) What is the nucleon number of P / Apakah nombor nukleon atom P?
35
(c) State the number of neutron in atom P / Nyatakan bilangan neutron atom P.
18
(d) State number of proton in atom P / Nyatakan bilangan proton atom P.
17
(e) (i) What is meant by isotope / Apakah maksud isotop?
Isotopes are atoms of the same element with same number of proton but different number of neutrons
18
Nilam Publication Sdn. Bhd.
01-Chem F4 (3p).indd 18 12/9/2011 5:59:33 PM