Page 86 - Science Class 6 Times Publication
P. 86
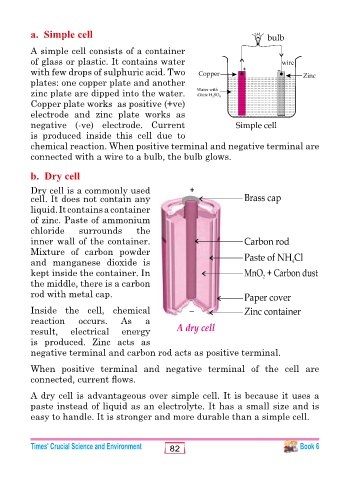
a. Simple cell
A simple cell consists of a container
of glass or plastic. It contains water wire
with few drops of sulphuric acid. Two
plates: one copper plate and another
zinc plate are dipped into the water.
Copper plate works as positive (+ve)
electrode and zinc plate works as
negative (-ve) electrode. Current
is produced inside this cell due to
chemical reaction. When positive terminal and negative terminal are
connected with a wire to a bulb, the bulb glows.
b. Dry cell
Dry cell is a commonly used
cell. It does not contain any
liquid. It contains a container
of zinc. Paste of ammonium
chloride surrounds the
inner wall of the container.
Mixture of carbon powder
and manganese dioxide is
kept inside the container. In
the middle, there is a carbon
rod with metal cap.
Inside the cell, chemical
reaction occurs. As a
result, electrical energy
is produced. Zinc acts as
negative terminal and carbon rod acts as positive terminal.
When positive terminal and negative terminal of the cell are
connected, current ows.
A dry cell is advantageous over simple cell. It is because it uses a
paste instead of liquid as an electrolyte. It has a small size and is
easy to handle. It is stronger and more durable than a simple cell.
Times' Crucial Science and Environment 82 Book 6