Page 9 - Electronic Configuration
P. 9
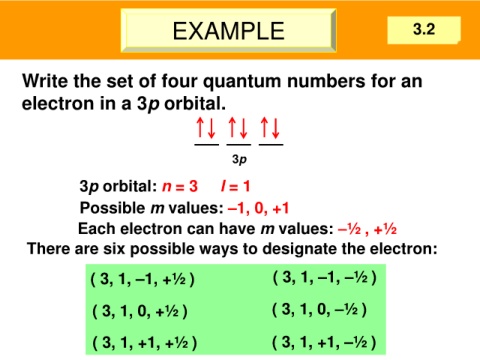
EXAMPLE 3.2
Write the set of four quantum numbers for an
electron in a 3p orbital.
3p
3p orbital: n = 3 l = 1
Possible m values: –1, 0, +1
Each electron can have m values: –½ , +½
There are six possible ways to designate the electron:
( 3, 1, –1, +½ ) ( 3, 1, –1, –½ )
( 3, 1, 0, +½ ) ( 3, 1, 0, –½ )
( 3, 1, +1, +½ ) ( 3, 1, +1, –½ )