Page 173 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 173
+
SPM Chemistry A xplosive Module (Chemistry A Ticket For “U”) ANSWERS
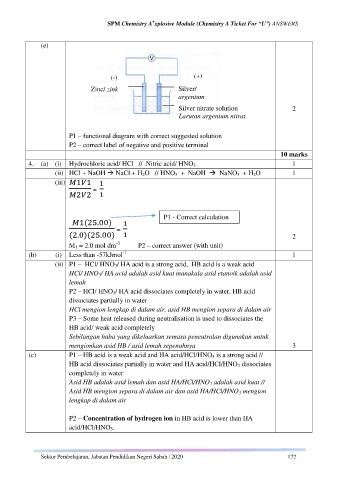
(e)
(+)
(-)
Zinc/ zink Silver/
argentum
Silver nitrate solution 2
Larutan argentum nitrat
P1 – functional diagram with correct suggested solution
P2 – correct label of negative and positive terminal
10 marks
4. (a) (i) Hydrochloric acid/ HCl // Nitric acid/ HNO 3 1
(ii) HCl + NaOH NaCl + H 2O // HNO 3 + NaOH NaNO 3 + H 2O 1
(iii)
=
P1 - Correct calculation
=
2
-3
M 1 = 2.0 mol dm P2 – correct answer (with unit)
(b) (i) Less than -57kJmol -1 1
(ii) P1 – HCl/ HNO 3/ HA acid is a strong acid, HB acid is a weak acid
HCl/ HNO 3/ HA acid adalah asid kuat manakala asid etanoik adalah asid
lemah
P2 – HCl/ HNO 3/ HA acid dissociates completely in water, HB acid
dissociates partially in water
HCl mengion lengkap di dalam air, asid HB mengion separa di dalam air
P3 – Some heat released during neutralisation is used to dissociates the
HB acid/ weak acid completely
Sebilangan haba yang dikeluarkan semasa peneutralan digunakan untuk
mengionkan asid HB / asid lemah sepenuhnya 3
(c) P1 – HB acid is a weak acid and HA acid/HCl/HNO 3 is a strong acid //
HB acid dissociates partially in water and HA acid/HCl/HNO 3 dissociates
completely in water
Asid HB adalah asid lemah dan asid HA/HCl/HNO 3 adalah asid kuat //
Asid HB mengion separa di dalam air dan asid HA/HCl/HNO 3 mengion
lengkap di dalam air
P2 – Concentration of hydrogen ion in HB acid is lower than HA
acid/HCl/HNO 3.
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 172