Page 175 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 175
+
SPM Chemistry A xplosive Module (Chemistry A Ticket For “U”) ANSWERS
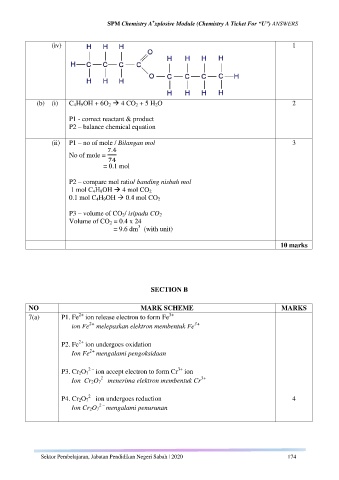
(iv) 1
(b) (i) C 4H 9OH + 6O 2 4 CO 2 + 5 H 2O 2
P1 - correct reactant & product
P2 – balance chemical equation
(ii) P1 – no of mole / Bilangan mol 3
No of mole =
= 0.1 mol
P2 – compare mol ratio/ banding nisbah mol
1 mol C 4H 9OH 4 mol CO 2
0.1 mol C 4H 9OH 0.4 mol CO 2
P3 – volume of CO 2/ isipadu CO 2
Volume of CO 2 = 0.4 x 24
3
= 9.6 dm (with unit)
10 marks
SECTION B
NO MARK SCHEME MARKS
3+
2+
7(a) P1. Fe ion release electron to form Fe
3+
2+
ion Fe melepaskan elektron membentuk Fe
2+
P2. Fe ion undergoes oxidation
2+
Ion Fe mengalami pengoksidaan
3+
2 –
P3. Cr 2O 7 ion accept electron to form Cr ion
2 – 3+
Ion Cr 2O 7 menerima elektron membentuk Cr
2 –
P4. Cr 2O 7 ion undergoes reduction 4
2 –
Ion Cr 2O 7 mengalami penurunan
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 174