Page 86 - MODUL KIMIA A+XPLOSIVE SPM (LATEST)
P. 86
YELLOW ZONE [YZ]
(ii) Explain why dry hydrogen gas was continuously flowed when the product was
cooled at room temperature.
Terangkan mengapa gas hidrogen kering terus dialirkan semasa hasil disejukkan
pada suhu bilik.
_________________________________________________________________
[1M]
(iii) How do you ensure all lead oxide have completely reacted?
Bagaimanakah anda memastikan semua plumbum oksida telah bertindak balas
dengan lengkap?
_________________________________________________________________
[1M]
(iv) Write a balanced chemical equation for the reaction involved in the determination
of empirical formula above.
Tuliskan persamaan kimia seimbang untuk tindak balas yang terlibat dalam
penentuan formula empirik di atas.
_________________________________________________________________
[1M]
(v) Can the empirical formula for zinc oxide be determined by using this method?
Explain your answer.
Bolehkah formula empirik bagi zink oksida ditentukan dengan menggunakan
kaedah ini?
Terangkan jawapan anda.
_________________________________________________________________
[1M]
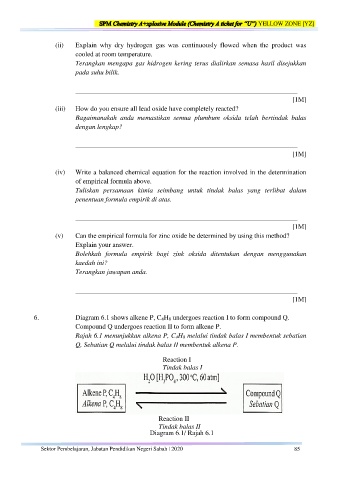
6. Diagram 6.1 shows alkene P, C 4H 8 undergoes reaction I to form compound Q.
Compound Q undergoes reaction II to form alkene P.
Rajah 6.1 menunjukkan alkena P, C 4H 8 melalui tindak balas I membentuk sebatian
Q, Sebatian Q melalui tindak balas II membentuk alkena P.
Reaction I
Tindak balas I
Reaction II
Tindak balas II
Diagram 6.1/ Rajah 6.1
Sektor Pembelajaran, Jabatan Pendidikan Negeri Sabah | 2020 85