Page 130 - Physics Form 5 KSSM_Neat
P. 130
Results:
Table 3.14
Dry cells in series Dry cells in parallel
e.m.f., Ԑ / V
Potential difference, V / V
KEMENTERIAN PENDIDIKAN MALAYSIA
Current, I / A
Effective internal resistance, r / Ω
e
Discussion:
Which arrangement of dry cells produces smaller effective internal resistance? Explain.
Alkaline batteries that use potassium hydroxide electrolyte are twice as durable compared to
zinc-carbon batteries that use ammonia chloride. Different electrolyte cause the internal resistance
of the two batteries to be different. Apart from the electrolyte used, the arrangements of dry cells
also affect the effective internal resistance.
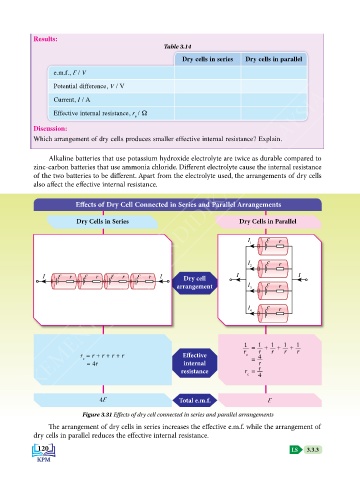
Effects of Dry Cell Connected in Series and Parallel Arrangements
Dry Cells in Series Dry Cells in Parallel
I Ɛ r
1
I Ɛ r
2
I Ɛ r Ɛ r Ɛ r Ɛ r I Dry cell I I
arrangement I 3 Ɛ r
I Ɛ r
4
1
1
1
1 = + + + 1
r = r + r + r + r Effective r e 4 r r r r
e = 4r internal = r
resistance r = r
e 4
4Ԑ Total e.m.f. Ԑ
Figure 3.31 Effects of dry cell connected in series and parallel arrangements
The arrangement of dry cells in series increases the effective e.m.f. while the arrangement of
dry cells in parallel reduces the effective internal resistance.
120 LS 3.3.3