Page 221 - Physics Form 5 KSSM_Neat
P. 221
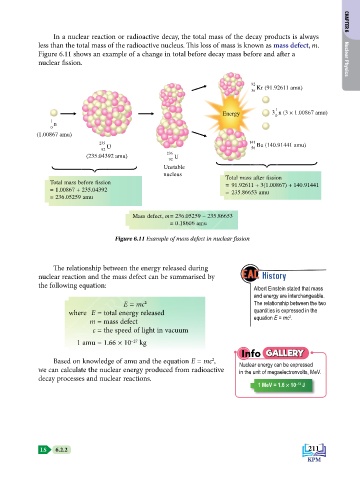
In a nuclear reaction or radioactive decay, the total mass of the decay products is always CHAPTER 6
less than the total mass of the radioactive nucleus. This loss of mass is known as mass defect, m.
Figure 6.11 shows an example of a change in total before decay mass before and after a
nuclear fission. Nuclear Physics
92
KEMENTERIAN PENDIDIKAN MALAYSIA
Kr (91.92611 amu)
36
1
Energy 3 n (3 × 1.00867 amu)
0
1
n
0
(1.00867 amu)
235 141 Ba (140.91441 amu)
U 56
92
236
(235.04392 amu) U
92
Unstable
nucleus
Total mass after fission
Total mass before fission
= 91.92611 + 3(1.00867) + 140.91441
= 1.00867 + 235.04392
= 235.86653 amu
= 236.05259 amu
Mass defect, m = 236.05259 – 235.86653
= 0.18606 amu
Figure 6.11 Example of mass defect in nuclear fission
The relationship between the energy released during
nuclear reaction and the mass defect can be summarised by History
the following equation: Albert Einstein stated that mass
and energy are interchangeable.
E = mc 2 The relationship between the two
where E = total energy released quantities is expressed in the
2
m = mass defect equation E = mc .
c = the speed of light in vacuum
1 amu = 1.66 × 10 kg
–27
Info GALLERY
Based on knowledge of amu and the equation E = mc , Nuclear energy can be expressed
2
we can calculate the nuclear energy produced from radioactive in the unit of megaelectronvolts, MeV.
decay processes and nuclear reactions.
1 MeV = 1.6 µ 10 J
–13
LS 6.2.2 211