Page 2249 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2249
1996 Part XII Hemostasis and Thrombosis
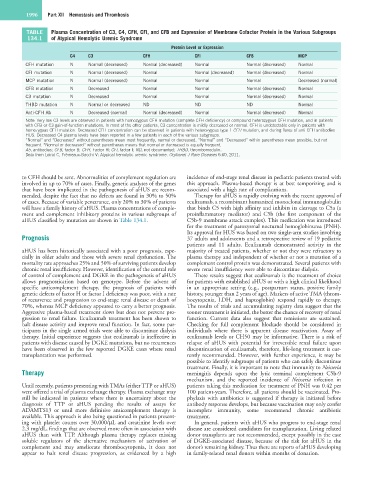
TABLE Plasma Concentration of C3, C4, CFH, CFI, and CFB and Expression of Membrane Cofactor Protein in the Various Subgroups
134.1 of Atypical Hemolytic Uremic Syndrome
Protein Level or Expression
C4 C3 CFH CFI CFB MCP
CFH mutation N Normal (decreased) Normal (decreased) Normal Normal (decreased) Normal
CFI mutation N Normal (decreased) Normal Normal (decreased) Normal (decreased) Normal
MCP mutation N Normal (decreased) Normal Normal Normal Decreased (normal)
CFB mutation N Decreased Normal Normal Normal (decreased) Normal
C3 mutation N Decreased Normal Normal Normal (decreased) Normal
THBD mutation N Normal or decreased ND ND ND Normal
Anti-CFH Ab N Decreased (normal) Normal (decreased) Normal Normal (decreased) Normal
Note: Very low C3 levels are observed in patients with homozygous CFH mutation (complete CFH deficiency) or compound heterozygous CFH mutation, and in patients
with CFB or C3 gain-of-function mutations. In most of the other patients, C3 concentration is mildly decreased or normal. CFH is undetectable only in patients with
homozygous CFH mutation. Decreased CFH concentration can be observed in patients with heterozygous type 1 CFH mutation, and during flares of anti-CFH antibodies-
HUS. Decreased C4 plasma levels have been reported in a few patients in each of the various subgroups.
“Normal” and “Decreased” without parentheses mean most frequently, normal or decreased. “Normal” and “Decreased” within parentheses mean possible, but not
frequent. “Normal or decreased” without parentheses means that normal or decreased is equally frequent.
Ab, antibodies; CFB, factor B; CFH, Factor H; CFI, factor I; ND, not documented; THBD, thrombomodulin.
Data from Loirat C, Frémeaux-Bacchi V: Atypical hemolytic uremic syndrome. Orphanet J Rare Diseases 6:60, 2011.
to CFH should be sent. Abnormalities of complement regulation are incidence of end-stage renal disease in pediatric patients treated with
involved in up to 70% of cases. Finally, genetic analyses of the genes this approach. Plasma-based therapy is at best temporizing and is
that have been implicated in the pathogenesis of aHUS are recom- associated with a high rate of complications.
mended, despite the fact that no defects are found in 30% to 50% Therapy for aHUS is rapidly evolving with the recent approval of
of cases. Because of variable penetrance, only 20% to 30% of patients eculizumab, a recombinant humanized monoclonal immunoglobulin
will have a family history of aHUS. Plasma concentrations of comple- that binds C5 with high affinity and inhibits its cleavage to C5a (a
ment and complement inhibitory proteins in various subgroups of proinflammatory mediator) and C5b (the first component of the
aHUS classified by mutation are shown in Table 134.1. C5b-9 membrane attack complex). This medication was introduced
for the treatment of paroxysmal nocturnal hemoglobinurea (PNH).
Its approval for HUS was based on two single-arm studies involving
Prognosis 37 adults and adolescents and a retrospective review of 19 pediatric
patients and 11 adults. Eculizumab demonstrated activity in the
aHUS has been historically associated with a poor prognosis, espe- majority of treated patients, whether or not they were refractory to
cially in older adults and those with severe renal dysfunction. The plasma therapy and independent of whether or not a mutation of a
mortality rate approaches 25% and 50% of surviving patients develop complement control protein was demonstrated. Several patients with
chronic renal insufficiency. However, identification of the central role severe renal insufficiency were able to discontinue dialysis.
of control of complement and DGKE in the pathogenesis of aHUS These results suggest that eculizumab is the treatment of choice
allows prognostication based on genotype. Before the advent of for patients with established aHUS or with a high clinical likelihood
specific anticomplement therapy, the prognosis of patients with in an appropriate setting (e.g., postpartum status, positive family
genetic defects of factor H or factor I deficiency was poor, with a rate history, younger than 2 years of age). Markers of active TMA (throm-
of recurrence and progression to end-stage renal disease or death of bocytopenia, LDH, and haptoglobin) respond rapidly to therapy.
70%, whereas MCP deficiency appeared to carry a better prognosis. The results of trials and accumulating registry data suggest that the
Aggressive plasma-based treatment slows but does not prevent pro- sooner treatment is initiated, the better the chance of recovery of renal
gression to renal failure. Eculizumab treatment has been shown to function. Current data also suggest that remissions are sustained.
halt disease activity and improve renal function. In fact, some par- Checking for full complement blockade should be considered in
ticipants in the single armed trials were able to discontinue dialysis individuals where there is apparent disease reactivation. Assay of
therapy. Initial experience suggests that eculizumab is ineffective in eculizumab levels or CH50 may be informative. There is a risk of
patients with disease caused by DGKE mutations, but no recurrences relapse of aHUS with potential for irreversible renal failure upon
have been observed in the few reported DGKE cases where renal discontinuation of eculizumab, therefore, life-long treatment is cur-
transplantation was performed. rently recommended. However, with further experience, it may be
possible to identify subgroups of patients who can safely discontinue
treatment. Finally, it is important to note that immunity to Neisseria
Therapy meningitis depends upon the lytic terminal complement C5b-9
mechanism, and the reported incidence of Neisseria infection in
Until recently, patients presenting with TMAs (either TTP or aHUS) patients taking this medication for treatment of PNH was 0.42 per
were offered a trial of plasma exchange therapy. Plasma exchange may 100 patient-years. Therefore, all patients should be vaccinated. Pro-
still be indicated in patients where there is uncertainty about the phylaxis with antibiotics is suggested if therapy is initiated before
diagnosis of TTP or aHUS pending the results of assays for antibody response develops, but because vaccination may only confer
ADAMTS13 or until more definitive anticomplement therapy is incomplete immunity, some recommend chronic antibiotic
available. This approach is also being questioned in patients present- treatment.
ing with platelet counts over 30,000/µL and creatinine levels over In general, patients with aHUS who progress to end-stage renal
2.3 mg/dL, findings that are observed more often in association with disease are considered candidates for transplantation. Living related
aHUS than with TTP. Although plasma therapy replaces missing donor transplants are not recommended, except possibly in the case
soluble regulators of the alternative mechanism of activation of of DGKE-associated disease, because of the risk for aHUS in the
complement and may ameliorate thrombocytopenia, it does not donor’s remaining kidney. Thus there are reports of aHUS developing
appear to halt renal disease progression, as evidenced by a high in family-related renal donors within months of donation.