Page 1869 - Williams Hematology ( PDFDrive )
P. 1869
1844 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1845
A E
B F
C D G
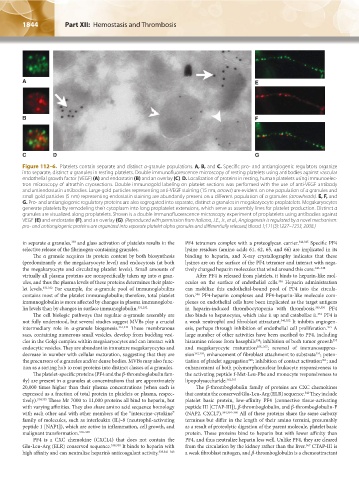
Figure 112–6. Platelets contain separate and distinct α-granule populations. A, B, and C. Specific pro- and antiangiogenic regulators organize
into separate, distinct α granules in resting platelets. Double immunofluorescence microscopy of resting platelets using antibodies against vascular
endothelial growth factor (VEGF) (A) and endostatin (B) and an overlay (C). D. Localization of proteins in resting, human platelets using immunoelec-
tron microscopy of ultrathin cryosections. Double immunogold labeling on platelet sections was performed with the use of anti-VEGF antibody
and antiendostatin antibodies. Large gold particles representing anti-VEGF staining (15 nm, arrows) are evident on one population of α granules and
small gold particles (5 nm) representing endostatin staining are abundantly present on a different population of α granules (arrowheads). E, F, and
G. Pro- and antiangiogenic regulatory proteins are also segregated into separate, distinct α granules in megakaryocyte proplatelets. Megakaryocytes
generate platelets by remodeling their cytoplasm into long proplatelet extensions, which serve as assembly lines for platelet production. Distinct α
granules are visualized along proplatelets. Shown is a double immunofluorescence microscopy experiment of proplatelets using antibodies against
VEGF (E) and endostatin (F), and an overlay (G). (Reproduced with permission from Italiano, J.E., Jr., et al., Angiogenesis is regulated by a novel mechanism:
pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released, Blood 1;111(3):1227–1233, 2008.)
in separate α granules, and glass activation of platelets results in the PF4 tetramers complex with a proteoglycan carrier. 344,345 Specific PF4
330
selective release of the fibrinogen-containing granules. lysine residues (amino acids 61, 62, 65, and 66) are implicated in its
The α granule acquires its protein content by both biosynthesis binding to heparin, and X-ray crystallography indicates that these
(predominantly at the megakaryocyte level) and endocytosis (at both lysines are on the surface of the PF4 tetramer and interact with nega-
the megakaryocyte and circulating platelet levels). Small amounts of tively charged heparin molecules that wind around this core. 346–348
virtually all plasma proteins are nonspecifically taken up into α gran- After PF4 is released from platelets, it binds to heparin-like mol-
ules, and thus the plasma levels of these proteins determines their plate- ecules on the surface of endothelial cells. Heparin administration
346
let levels. 331,332 For example, the α-granule pool of immunoglobulins can mobilize this endothelial-bound pool of PF4 into the circula-
contains most of the platelet immunoglobulin; therefore, total platelet tion. PF4-heparin complexes and PF4-heparin-like molecule com-
346
immunoglobulin is more affected by changes in plasma immunoglobu- plexes on endothelial cells have been implicated as the target antigens
lin levels than by changes in surface immunoglobulin. 331,332 in heparin-induced thrombocytopenia with thrombosis. 349,350 PF4
The cell biologic pathways that regulate α-granule assembly are also binds to hepatocytes, which take it up and catabolize it. PF4 is
351
not fully understood, but several studies suggest MVBs play a crucial a weak neutrophil and fibroblast attractant. 340,352 It inhibits angiogen-
intermediary role in α-granule biogenesis. 316,333 These membranous esis, perhaps through inhibition of endothelial cell proliferation. A
353
sacs, containing numerous small vesicles, develop from budding vesi- large number of other activities have been ascribed to PF4, including
cles in the Golgi complex within megakaryocytes and can interact with histamine release from basophils ; inhibition of both tumor growth
353
354
endocytic vesicles. They are abundant in immature megakaryocytes and and megakaryocyte maturation 355–357 ; reversal of immunosuppres-
decrease in number with cellular maturation, suggesting that they are sion 352,358 ; enhancement of fibroblast attachment to substrata ; poten-
359
the precursors of α granules and/or dense bodies. MVBs may also func- tiation of platelet aggregation ; inhibition of contact activation ; and
360
361
tion as a sorting hub to rout proteins into distinct classes of α granules. enhancement of both polymorphonuclear leukocyte responsiveness to
The platelet-specific proteins (PF4 and the β-thromboglobulin fam- the activating peptide f-Met-Leu-Phe and monocyte responsiveness to
ily) are present in α granules at concentrations that are approximately lipopolysaccharide. 362,363
20,000 times higher than their plasma concentrations (when each is The β-thromboglobulin family of proteins are CXC chemokines
expressed as a fraction of total protein in platelets or plasma, respec- that contain the conserved Glu-Leu-Arg (ELR) sequence. They include
340
tively). 334,335 These Mr 7000 to 11,000 proteins all bind to heparin, but platelet basic protein, low-affinity PF4 (connective tissue-activating
with varying affinities. They also share amino acid sequence homology peptide III [CTAP-III]), β-thromboglobulin, and β-thromboglobulin-F
with each other and with other members of the “intercrine-cytokine” (NAP2, CXCL7). 334,364–366 All of these proteins share the same carboxy
family of molecules, such as interleukin (IL)-8 (neutrophil-activating terminus but differ in the length of their amino termini, presumably
peptide 1 [NAP1]), which are active in inflammation, cell growth, and as a result of proteolytic digestion of the parent molecule, platelet basic
malignant transformation. 336–338 protein. These proteins bind to heparin but with lower affinity than
PF4 is a CXC chemokine (CXCL4) that does not contain the PF4, and thus neutralize heparin less well. Unlike PF4, they are cleared
Glu-Leu-Arg (ELR) conserved sequence. 339,340 It binds to heparin with from the circulation by the kidney rather than the liver. CTAP-III is
367
high affinity and can neutralize heparin’s anticoagulant activity. 335,341–343 a weak fibroblast mitogen, and β-thromboglobulin is a chemoattractant
Kaushansky_chapter 112_p1829-1914.indd 1844 17/09/15 3:26 pm