Page 1864 - Williams Hematology ( PDFDrive )
P. 1864
1838 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1839
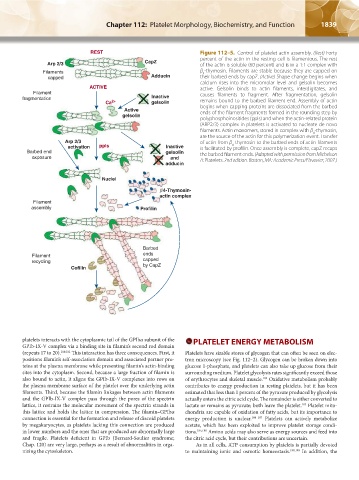
Figure 112–5. Control of platelet actin assembly. (Rest) Forty
percent of the actin in the resting cell is filamentous. The rest
of the actin is soluble (60 percent) and is in a 1:1 complex with
β -thymosin. Filaments are stable because they are capped on
4
their barbed ends by capZ. (Active) Shape change begins when
calcium rises into the micromolar level and gelsolin becomes
active. Gelsolin binds to actin filaments, interdigitates, and
causes filaments to fragment. After fragmentation, gelsolin
remains bound to the barbed filament end. Assembly of actin
begins when capping proteins are dissociated from the barbed
ends of the filament fragments formed in the rounding step by
polyphosphoinositides (ppIs) and when the actin-related protein
(ARP2/3) complex in platelets is activated to nucleate de novo
filaments. Actin monomers, stored in complex with β -thymosin,
4
are the source of the actin for this polymerization event. Transfer
of actin from β -thymosin to the barbed ends of actin filaments
4
is facilitated by profilin. Once assembly is complete, capZ recaps
the barbed filament ends. (Adapted with permission from Michelson
A: Platelets. 2nd edition. Boston, MA: Academic Press/Elsvevier; 2007.)
platelets interacts with the cytoplasmic tail of the GPIbα subunit of the PLATELET ENERGY METABOLISM
GPIb-IX-V complex via a binding site in filamin’s second rod domain
(repeats 17 to 20). 180,181 This interaction has three consequences. First, it Platelets have sizable stores of glycogen that can often be seen on elec-
positions filamin’s self-association domain and associated partner pro- tron microscopy (see Fig. 112–2). Glycogen can be broken down into
teins at the plasma membrane while presenting filamin’s actin-binding glucose 1-phosphate, and platelets can also take up glucose from their
sites into the cytoplasm. Second, because a large fraction of filamin is surrounding medium. Platelet glycolysis rates significantly exceed those
also bound to actin, it aligns the GPIb-IX-V complexes into rows on of erythrocytes and skeletal muscle. Oxidative metabolism probably
182
the plasma membrane surface of the platelet over the underlying actin contributes to energy production in resting platelets, but it has been
filaments. Third, because the filamin linkages between actin filaments estimated that less than 1 percent of the pyruvate produced by glycolysis
and the GPIb-IX-V complex pass through the pores of the spectrin actually enters the citric acid cycle. The remainder is either converted to
lattice, it restrains the molecular movement of the spectrin strands in lactate or remains as pyruvate; both leave the platelet. Platelet mito-
183
this lattice and holds the lattice in compression. The filamin–GPIbα chondria are capable of oxidation of fatty acids, but its importance to
connection is essential for the formation and release of discoid platelets energy production is unclear. 184–187 Platelets can actively metabolize
by megakaryocytes, as platelets lacking this connection are produced acetate, which has been exploited to improve platelet storage condi-
in lower numbers and the ones that are produced are abnormally large tions. 185,188 Amino acids may also serve as energy sources and feed into
and fragile. Platelets deficient in GPIb (Bernard-Soulier syndrome; the citric acid cycle, but their contributions are uncertain.
Chap. 120) are very large, perhaps as a result of abnormalities in orga- As in all cells, ATP consumption by platelets is partially devoted
nizing the cytoskeleton. to maintaining ionic and osmotic homeostasis. 189,190 In addition, the
Kaushansky_chapter 112_p1829-1914.indd 1839 17/09/15 3:26 pm