Page 1984 - Williams Hematology ( PDFDrive )
P. 1984
1958 Part XII: Hemostasis and Thrombosis Chapter 114: Control of Coagulation Reactions 1959
product and regenerates the active site Ser residue of the protease. one of which (Asn135) is variably glycosylated, giving rise to a β-i-
However, serpins have an ability to undergo major conformational soform that has higher affinity for heparin. 317,318 Heparin binding to
changes following cleavage at the reactive site residue that can distort antithrombin is mediated by a number of positively charged Arg and
that protease’s active site region and lock the enzyme into the protease– Lys residues in the N-terminal region of the molecule, including Lys11,
serpin complex in which both the serpin and the protease are essentially Arg13, Arg47, Lys114, Lys125, and Arg129, whereas the reactive center
deformed. 304–307,309–312 The dominant structural feature of native serpins loop containing the scissile peptide bond at Arg393-Ser394 is near the
is a large five-stranded β-sheet that defines the structure of an ellipsoidal C-terminus. 311
protein. Following cleavage at the reactive residue in the reactive center
loop by a protease, this extended loop is able to partially or completely ANTITHROMBIN GENE
insert itself into the five-stranded β-sheet, forming a very stable six The antithrombin gene comprising seven exons and six introns spans
-stranded β-sheet. If this insertion reaction proceeds before deacylation 13.4 kb and is located on chromosome 1q23–25 (see Table 114–1). 319,320
occurs, then the protease remains covalently attached to the reactive
center P1 residue through the protease’s active site Ser residue, and a sta- ANTITHROMBIN MUTATIONS
ble covalent protease–inhibitor complex with each protein in an altered
conformation is formed. 307,308 Hereditary deficiencies of antithrombin are risk factors for venous
Heparin enhancement of the rate of reaction between antithrombin thrombosis (Chap. 130). More than 100 different antithrombin muta-
and thrombin or other clotting factors is caused by two distinct effects tions are associated with thrombosis. An extensive database of muta-
321
of heparin, one involving conformational effects on antithrombin and tions is published and is available at http://www1.imperial.ac.uk/
the other involving “approximation” effects on both antithrombin and departmentofmedicine/divisions/experimentalmedicine/haematology/
thrombin. 300,307,308,311–315 For the first effect, a particular pentasaccharide coag/antithrombin/.
sequence within heparin binds antithrombin and potently causes a con- Mutations that cause antithrombin deficiency are scattered through-
formational change that converts antithrombin from its native state of out the gene. Molecular defects can be classified as type I, characterized
moderate reactivity to a conformation with relatively high reactivity. This by parallel decreases in antigen and activity, or type II, characterized
pentasaccharide contains a specific sulfated sequence of glucosamine by circulating dysfunctional molecules such that plasma has decreased
and iduronic acid residues, 300,307,308,311–315 and when it is present in a functional activity but normal or near-normal antigen levels. Type II
large heparin molecule, in low-molecular-weight heparin, or in a syn- defects are further classified based on whether the dysfunction involves
thetic pentasaccharide, it alters antithrombin conformation and greatly only reactive center defects that can be tested in the absence of hepa-
accelerates the reaction of antithrombin, especially with factor Xa. Syn- rin, only heparin-binding defects that can be tested only in the presence
thetic pentasaccharides, such as fondaparinux, which are analogues of of heparin, or both of these defects (pleiotropic effects). Reactive center
the naturally occurring sequence, are often termed to be indirect factor defects carry the largest risk of thrombosis, whereas heparin-binding
Xa inhibitors and have significant clinical utility. For the second mech- defects are associated with less risk of venous thrombosis (Chap. 130).
anistic effect, namely the approximation effect, unfractionated heparin
or low-molecular-weight heparins simultaneously bind to antithrombin TISSUE FACTOR PATHWAY INHIBITOR
and the target protease to promote frequent and geometrically produc-
tive encounters between protease and inhibitor, thus increasing the reac- TFPI, also known as lipoprotein-associated coagulation inhibitor or extrinsic
tion rate. Heparan sulfates to some extent can also act in this manner. pathway inhibitor, has a predicted mature protein sequence of 276 residues
The mature antithrombin polypeptide chain contains 432-amino- and a Mr of 34,000. However, TFPI is a complex protein and has at least
acid residues after cleavage of a propeptide from a 464-amino-acid-resi- three isoforms in blood vessels. 277,301–303,322–327 There are two alternatively
316
due precursor. It has four sites for N-linked carbohydrate attachment, spliced forms of TFPI designated TFPIα and TFPIβ (Fig. 114–7). 323,324
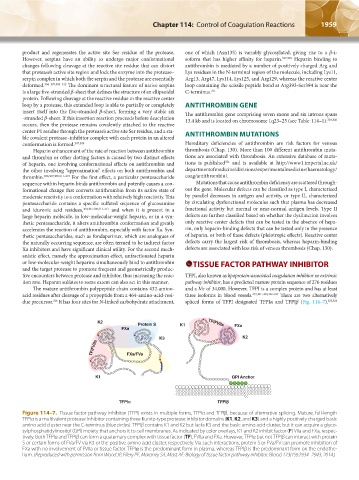
K2 Protein S K1 TF/FVIIa
FXa FXa
K3 K2
TF/FVIIa FXa/FVa
K1 GPI Anchor
TFPI` TFPIa
Figure 114–7. Tissue factor pathway inhibitor (TFPI) exists in multiple forms, TFPIα and TFPIβ, because of alternative splicing. Mature, full-length
TFPIα is a multivalent protease inhibitor containing three Kunitz-type protease inhibitor domains (K1, K2, and K3) and a highly positively charged basic
amino acid cluster near the C-terminus (blue circles). TFPIβ contains K1 and K2 but lacks K3 and the basic amino acid cluster, but it can acquire a glyco-
sylphosphatidylinositol (GPI) moiety that anchors it to cell membranes. As indicated by color overlays, K1 and K2 inhibit factor (F) VIIa and FXa, respec-
tively. Both TFPIα and TFPIβ can form a quaternary complex with tissue factor (TF), FVIIa and FXa. However, TFPIα but not TFPIβ can interact with protein
S or certain forms of FVa/FV via K3 or the positive amino acid cluster, respectively. Via such interactions, protein S or FVa/FV can promote inhibition of
FXa with no involvement of FVIIa or tissue factor. TFPIα is the predominant form in plasma, whereas TFPIβ is the predominant form on the endothe-
lium. (Reproduced with permission from Wood JP, Ellery PE, Maroney SA, Mast AE: Biology of tissue factor pathway inhibitor. Blood 123(19):2934–2943, 2014.)
Kaushansky_chapter 114_p1949-1966.indd 1959 9/18/15 10:06 AM