Page 2042 - Williams Hematology ( PDFDrive )
P. 2042
2016 Part XII: Hemostasis and Thrombosis Chapter 117: Thrombocytopenia 2017
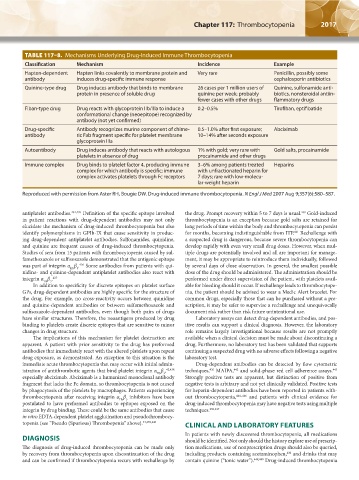
TABLE 117–8. Mechanisms Underlying Drug-Induced Immune Thrombocytopenia
Classification Mechanism Incidence Example
Hapten-dependent Hapten links covalently to membrane protein and Very rare Penicillin, possibly some
antibody induces drug-specific immune response cephalosporin antibiotics
Quinine-type drug Drug induces antibody that binds to membrane 26 cases per 1 million users of Quinine, sulfonamide anti-
protein in presence of soluble drug quinine per week; probably biotics, nonsteroidal antiin-
fewer cases with other drugs flammatory drugs
Fiban-type drug Drug reacts with glycoprotein IIb/IIIa to induce a 0.2–0.5% Tirofiban, eptifibatide
conformational change (neoepitope) recognized by
antibody (not yet confirmed)
Drug-specific Antibody recognizes murine component of chime- 0.5–1.0% after first exposure; Abciximab
antibody ric Fab fragment specific for platelet membrane 10–14% after seconds exposure
glycoprotein IIIa
Autoantibody Drug induces antibody that reacts with autologous 1% with gold; very rare with Gold salts, procainamide
platelets in absence of drug procainamide and other drugs
Immune complex Drug binds to platelet factor 4, producing immune 3–6% among patients treated Heparins
complex for which antibody is specific; immune with unfractionated heparin for
complex activates platelets through Fc receptors 7 days; rare with low-molecu-
lar-weight heparin
Reproduced with permission from Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007 Aug 9;357(6):580–587.
antiplatelet antibodies. 434,435 Definition of the specific epitope involved the drug. Prompt recovery within 5 to 7 days is usual. Gold-induced
430
in patient reactions with drug-dependent antibodies may not only thrombocytopenia is an exception because gold salts are retained for
elucidate the mechanism of drug-induced thrombocytopenia but also long periods of time within the body and thrombocytopenia can persist
identify polymorphisms in GPIb-IX that cause sensitivity in produc- for months, becoming indistinguishable from ITP. Rechallenge with
441
ing drug-dependent antiplatelet antibodies. Sulfonamides, quinidine, a suspected drug is dangerous, because severe thrombocytopenia can
and quinine are frequent causes of drug-induced thrombocytopenia. develop rapidly with even very small drug doses. However, when mul-
Studies of sera from 15 patients with thrombocytopenia caused by sul- tiple drugs are potentially involved and all are important for manage-
famethoxazole or sulfisoxazole demonstrated that the antigenic epitope ment, it may be appropriate to reintroduce them individually, followed
was part of integrin α β . Some antibodies from patients with qui- by several days of close observation. In general, the smallest possible
436
IIb 3
nidine- and quinine-dependent antiplatelet antibodies also react with dose of the drug should be administered. The administration should be
integrin α β . 437 performed under direct supervision of the patient, with platelets avail-
IIb 3
In addition to specificity for discrete epitopes on platelet surface able for bleeding should it occur. If rechallenge leads to thrombocytope-
GPs, drug-dependent antibodies are highly specific for the structure of nia, the patient should be advised to wear a Medic Alert bracelet. For
the drug. For example, no cross-reactivity occurs between quinidine common drugs, especially those that can be purchased without a pre-
and quinine-dependent antibodies or between sulfamethoxazole and scription, it may be safer to supervise a rechallenge and unequivocally
sulfisoxazole-dependent antibodies, even though both pairs of drugs document risk rather than risk future unintentional use.
have similar structures. Therefore, the neoantigens produced by drug Laboratory assays can detect drug-dependent antibodies, and pos-
binding to platelets create discrete epitopes that are sensitive to minor itive results can support a clinical diagnosis. However, the laboratory
changes in drug structure. role remains largely investigational because results are not promptly
The implications of this mechanism for platelet destruction are available when a clinical decision must be made about discontinuing a
apparent. A patient with prior sensitivity to the drug has preformed drug. Furthermore, no laboratory test has been validated that supports
antibodies that immediately react with the altered platelets upon repeat continuing a suspected drug with no adverse effects following a negative
drug exposure, as demonstrated. An exception to this situation is the laboratory test.
immediate acute thrombocytopenia that may occur with initial admin- Drug-dependent antibodies can be detected by flow cytometric
istration of antithrombotic agents that bind platelet integrin α β , 42,438 techniques, MAIPA, and solid-phase red cell adherence assays.
443
436
442
IIb 3
especially abciximab. Abciximab is a humanized monoclonal antibody Strongly positive tests are apparent, but distinction of positive from
fragment that lacks the Fc domain, so thrombocytopenia is not caused negative tests is arbitrary and not yet clinically validated. Positive tests
by phagocytosis of the platelets by macrophages. Patients experiencing for heparin-dependent antibodies have been reported in patients with-
thrombocytopenia after receiving integrin α β inhibitors have been out thrombocytopenia, 444–446 and patients with clinical evidence for
IIb 3
postulated to have preformed antibodies to epitopes exposed on the drug-induced thrombocytopenia may have negative tests using multiple
integrin by drug binding. These could be the same antibodies that cause techniques. 436,447
in vitro EDTA-dependent platelet agglutination and pseudothrombocy-
topenia (see “Pseudo (Spurious) Thrombopenia” above). 33,439,440 CLINICAL AND LABORATORY FEATURES
In patients with newly discovered thrombocytopenia, all medications
DIAGNOSIS should be identified. Not only should the history explore use of prescrip-
The diagnosis of drug-induced thrombocytopenia can be made only tion medications, use of nonprescription drugs should also be queried,
by recovery from thrombocytopenia upon discontinuation of the drug including products containing acetaminophen, and drinks that may
430
and can be confirmed if thrombocytopenia recurs with rechallenge by contain quinine (“tonic water”). 448,449 Drug-induced thrombocytopenia
Kaushansky_chapter 117_p1993-2024.indd 2017 9/21/15 2:33 PM