Page 289 - Williams Hematology ( PDFDrive )
P. 289
264 Part IV: Molecular and Cellular Hematology Chapter 18: Hematopoietic Stem Cells, Progenitors, and Cytokines 265
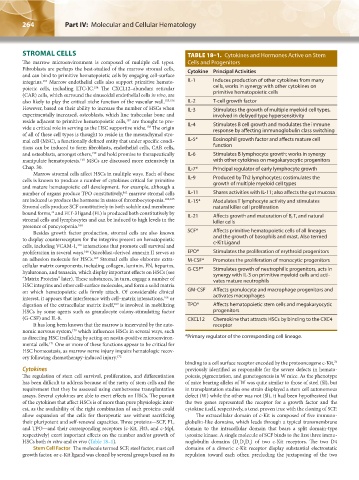
STROMAL CELLS TABLE 18–1. Cytokines and Hormones Active on Stem
The marrow microenvironment is composed of multiple cell types. Cells and Progenitors
Fibroblasts are perhaps the best-studied of the marrow stromal cells, Cytokine Principal Activities
and can bind to primitive hematopoietic cells by engaging cell-surface
integrins. Marrow endothelial cells also support primitive hemato- IL-1 Induces production of other cytokines from many
153
poietic cells, including LTC-IC. The CXCL12–abundant reticular cells, works in synergy with other cytokines on
154
(CAR) cells, which surround the sinusoidal endothelial cells in vivo, are primitive hematopoietic cells
also likely to play the critical niche function of the vascular wall. 155,156 IL-2 T-cell growth factor
However, based on their ability to increase the number of HSCs when IL-3 Stimulates the growth of multiple myeloid cell types,
experimentally increased, osteoblasts, which line trabecular bone and involved in delayed type hypersensitivity
reside adjacent to primitive hematopoietic cells, are thought to pro-
157
vide a critical role in serving as the HSC supportive niche. The origin IL-4 Stimulates B cell growth and modulates the immune
158
response by affecting immunoglobulin class switching
of all of these cell types is thought to reside in the mesenchymal stro-
mal cell (MSC), a functionally defined entity that under specific condi- IL-5* Eosinophil growth factor and affects mature cell
tions can be induced to form fibroblasts, endothelial cells, CAR cells, function
and osteoblasts, amongst others, and hold promise to therapeutically IL-6 Stimulates B lymphocyte growth; works in synergy
159
manipulate hematopoiesis. MSCs are discussed more extensively in with other cytokines on megakaryocytic progenitors
160
Chap. 30. IL-7* Principal regulator of early lymphocyte growth
Marrow stromal cells affect HSCs in multiple ways. Each of these
cells is known to produce a number of cytokines critical for primitive IL-9 Produced by Th2 lymphocytes; costimulates the
and mature hematopoietic cell development. For example, although a growth of multiple myeloid cell types
number of organs produce TPO constitutively, marrow stromal cells IL-11 Shares activities with IL-11; also affects the gut mucosa
161
are induced to produce the hormone in states of thrombocytopenia. 162,163 IL-15* Modulates T lymphocyte activity and stimulates
Stromal cells produce SCF constitutively in both soluble and membrane natural killer cell proliferation
bound forms, and FLT-3 ligand (FL) is produced both constitutively by IL-21 Affects growth and maturation of B, T, and natural
76
stromal cells and lymphocytes and can be induced to high levels in the killer cells
presence of pancytopenia. 164
Besides growth factor production, stromal cells are also known SCF* Affects primitive hematopoietic cells of all lineages
to display counterreceptors for the integrins present on hematopoietic and the growth of basophils and mast. Also termed
cells, including VCAM-1, interactions that promote cell survival and c-Kit Ligand
165
proliferation in several ways. Osteoblast-derived annexin II serves as EPO* Stimulates the proliferation of erythroid progenitors
166
an adhesion molecule for HSCs. Stromal cells also elaborate extra- M-CSF* Promotes the proliferation of monocytic progenitors
167
cellular matrix components, including collagen, laminin, FN, heparins,
hyaluronan, and tenascin, which display important effects on HSCs (see G-CSF* Stimulates growth of neutrophilic progenitors, acts in
synergy with IL-3 on primitive myeloid cells and acti-
“Matrix Proteins” later). These substances, in turn, engage a number of vates mature neutrophils
HSC integrins and other cell-surface molecules, and form a solid matrix
on which hematopoietic cells firmly attach. Of considerable clinical GM-CSF Affects granulocyte and macrophage progenitors and
interest, it appears that interference with cell–matrix interactions, or activates macrophages
168
digestion of the extracellular matrix itself, is involved in mobilizing TPO* Affects hematopoietic stem cells and megakaryocytic
169
HSCs by some agents such as granulocyte colony-stimulating factor progenitors
(G-CSF) and IL-8. CXCL12 Chemokine that attracts HSCs by binding to the CXC4
It has long been known that the marrow is innervated by the auto- receptor
nomic nervous system, which influences HSCs in several ways, such
170
as directing HSC trafficking by acting on nestin-positive microenviron- *Primary regulator of the corresponding cell lineage.
mental cells. One or more of these functions appear to be critical for
171
HSC homeostasis, as marrow nerve injury impairs hematologic recov-
ery following chemotherapy-induced injury. 172
binding to a cell surface receptor encoded by the protooncogene c-Kit,
76
Cytokines previously identified as responsible for the severe defects in hemato-
The regulation of stem cell survival, proliferation, and differentiation poiesis, pigmentation, and gametogenesis in W mice. As the phenotype
has been difficult to address because of the rarity of stem cells and the of mice bearing alleles of W was quite similar to those of steel (Sl), but
requirement that they be assessed using cumbersome transplantation in transplantation studies one strain displayed a stem cell autonomous
assays. Several cytokines are able to exert effects on HSCs. The pursuit defect (W) while the other was not (Sl), it had been hypothesized that
of the cytokines that affect HSCs is of more than pure physiologic inter- the two genes represented the receptor for a growth factor and the
est, as the availability of the right combination of such proteins could cytokine itself, respectively, a tenet proven true with the cloning of SCF.
allow expansion of the cells for therapeutic use without sacrificing The extracellular domain of c-Kit is composed of five immuno-
their pluripotent and self-renewal capacities. Three proteins—SCF, FL, globulin-like domains, which leads through a typical transmembrane
and TPO—and their corresponding receptors (c-Kit, Flt3, and c-Mpl, domain to the intracellular domain that bears a split domain-type
respectively) exert important effects on the number and/or growth of tyrosine kinase. A single molecule of SCF binds to the first three immu-
HSCs both in vitro and in vivo (Table 18–1). noglobulin domains (D D D ) of two c-Kit receptors. The two D4
2
3
1
Stem Cell Factor The molecule termed SCF, steel factor, mast cell domains of a dimeric c-Kit receptor display substantial electrostatic
growth factor, or c-Kit ligand was cloned by several groups based on its repulsion toward each other, precluding the juxtaposing of the two
Kaushansky_chapter 18_p0257-0278.indd 264 9/19/15 12:05 AM