Page 941 - Williams Hematology ( PDFDrive )
P. 941
916 Part VI: The Erythrocyte Chapter 59: Polyclonal and Hereditary Sideroblastic Anemias 917
TABLE 59–1. Classification of Sideroblastic Anemias given the present state of knowledge, to discuss both forms together.
The pathogenesis of the disorder may be viewed from two standpoints:
I. Acquired the underlying biochemical lesions and the mechanism(s) of the anemia
A. Primary sideroblastic anemia (myelodysplastic syndromes) itself.
(Chap. 88)
1. Subunit 1 of the mitochondrial cytochrome oxidase 54,55 Biochemical Lesions and Genetics
B. Sideroblastic anemia secondary to: In the search for the biochemical lesions responsible for the develop-
1. Isoniazid 21,22 ment of sideroblastic anemia, attention has been focused upon an intra-
mitochondrial defect in heme synthesis and on possible disturbances in
2. Pyrazinamide 21,22 pyridoxine metabolism.
3. Cycloserine 149
4. Chloramphenicol 149 Defects in Heme Synthesis
5. Ethanol 118 The role of defects in heme biosynthesis have occupied central stage
35
6. Lead 24 since the early studies of Garby and colleagues, who postulated that
7. Chronic neoplastic disease (Chap. 8) such a defect might exist; they demonstrated that the level of free ery-
throcyte protoporphyrin was decreased. Subsequently, a variety of
8. Zinc-induced copper deficiency 124,125 abnormalities of the levels of precursors and of their rate of incorpora-
II. Hereditary tion into heme was documented (Chap. 58). 36–41 However, the findings
A. X chromosome linked have not all been consistent, as levels of free erythrocyte protoporphyrin
B. Autosomal have often been increased, 42,43 not diminished. The role of mitochondria
1. Defects in the erythroid specific mitochondrial carrier in the etiology of sideroblastic anemia gained further credence when
family protein SLC25A38 47 mutations of the mitochondrial genome were found in patients with
15–19
2. Mitochondrial myopathy and sideroblastic anemia (PSU1 Pearson syndrome.
mutations) 57,108,109 Hereditary Sideroblastic Anemias
C. Mitochondrial Shortly after the identification of erythroid-specific ALA synthase
1. Pearson marrow-pancreas syndrome 15–19 (ALAS2, the first enzyme in heme synthesis; Fig. 59–2), it became
apparent that most patients with hereditary X-linked sideroblastic ane-
mias (XLSAs) had mutations in the ALAS2 gene. 44–46 However, a pro-
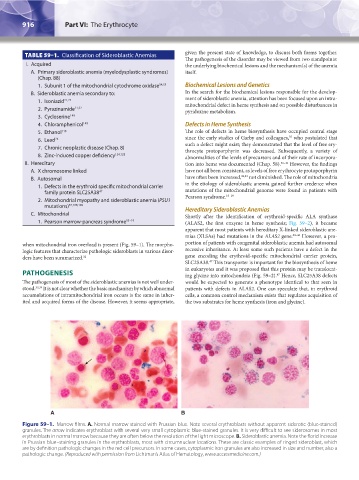
when mitochondrial iron overload is present (Fig. 59–1). The morpho- portion of patients with congenital sideroblastic anemia had autosomal
logic features that characterize pathologic sideroblasts in various disor- recessive inheritance. At least some such patients have a defect in the
ders have been summarized. 32 gene encoding the erythroid-specific mitochondrial carrier protein,
SLC25A38. This transporter is important for the biosynthesis of heme
47
PATHOGENESIS in eukaryotes and it was proposed that this protein may be translocat-
ing glycine into mitochondria (Fig. 59–2). Hence, SLC25A38 defects
47
The pathogenesis of most of the sideroblastic anemias is not well under- would be expected to generate a phenotype identical to that seen in
stood. 33,34 It is not clear whether the basic mechanism by which abnormal patients with defects in ALAS2. One can speculate that, in erythroid
accumulations of intramitochondrial iron occurs is the same in inher- cells, a common control mechanism exists that regulates acquisition of
ited and acquired forms of the disease. However, it seems appropriate, the two substrates for heme synthesis (iron and glycine).
A B
Figure 59–1. Marrow films. A. Normal marrow stained with Prussian blue. Note several erythroblasts without apparent siderotic (blue-stained)
granules. The arrow indicates erythroblast with several very small cytoplasmic blue-stained granules. It is very difficult to see siderosomes in most
erythroblasts in normal marrow because they are often below the resolution of the light microscope. B. Sideroblastic anemia. Note the florid increase
in Prussian blue–staining granules in the erythroblasts, most with circumnuclear locations. These are classic examples of ringed sideroblast, which
are by definition pathologic changes in the red cell precursors. In some cases, cytoplasmic iron granules are also increased in size and number, also a
pathologic change. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
Kaushansky_chapter 59_p0915-0922.indd 916 9/17/15 3:17 PM