Page 1112 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1112
1076 ParT EIGhT Immunology of Neoplasia
Please check your eBook at https://expertconsult.inkling.com/
for self-assessment questions. See inside cover for registration
details.
REFERENCES
1. Nogai H, Dorken B, Lenz G. Pathogenesis of non-Hodgkin’s lymphoma. J
Clin Oncol 2011;29:1803–11.
2. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours
of haematopoietic and lymphoid tissues. 4th ed. Lyon, France:
International Agency for Research on Cancer; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World
Health Organization (WHO) classification of lymphoid neoplasms. Blood
2016;pii: blood-2016-01-643569.
4. Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification
of lymphoid neoplasms and beyond: evolving concepts and practical
applications. Blood 2011;1175:19–32.
5. Treon SP, Xu L, Yang G, et al. MYD88 L265P Somatic mutation in
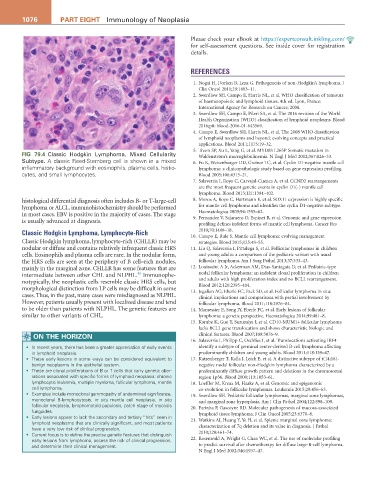
FIG 79.4 Classic Hodgkin Lymphoma, Mixed Cellularity Waldenström’s macroglobulinemia. N Engl J Med 2012;367:826–33.
Subtype. A classic Reed-Sternberg cell is shown in a mixed 6. Fu K, Weisenburger DD, Greiner TC, et al. Cyclin D1-negative mantle cell
inflammatory background with eosinophils, plasma cells, histio- lymphoma: a clinicopathologic study based on gene expression profiling.
cytes, and small lymphocytes. Blood 2005;106:4315–21.
7. Salaverria I, Royo C, Carvajal-Cuenca A, et al. CCND2 rearrangements
are the most frequent genetic events in cyclin D1(-) mantle cell
lymphoma. Blood 2013;121:1394–402.
histological differential diagnosis often includes B- or T-large-cell 8. Mozos A, Royo C, Hartmann E, et al. SOX11 expression is highly specific
lymphoma or ALCL, immunohistochemistry should be performed for mantle cell lymphoma and identifies the cyclin D1-negative subtype.
in most cases. EBV is positive in the majority of cases. The stage Haematologica 2009;94:1555–62.
is usually advanced at diagnosis. 9. Fernandez V, Salamero O, Espinet B, et al. Genomic and gene expression
profiling defines indolent forms of mantle cell lymphoma. Cancer Res
Classic Hodgkin Lymphoma, Lymphocyte-Rich 2010;70:1408–18.
10. Campo E, Rule S. Mantle cell lymphoma: evolving management
Classic Hodgkin lymphoma, lymphocyte-rich (CHLLR) may be strategies. Blood 2015;125:48–55.
nodular or diffuse and contains relatively infrequent classic HRS 11. Liu Q, Salaverria I, Pittaluga S, et al. Follicular lymphomas in children
cells. Eosinophils and plasma cells are rare. In the nodular form, and young adults: a comparison of the pediatric variant with usual
the HRS cells are seen at the periphery of B cell–rich nodules, follicular lymphoma. Am J Surg Pathol 2013;37:333–43.
mainly in the marginal zone. CHLLR has some features that are 12. Louissaint A Jr, Ackerman AM, Dias-Santagata D, et al. Pediatric-type
45
intermediate between other CHL and NLPHL. Immunophe- nodal follicular lymphoma: an indolent clonal proliferation in children
notypically, the neoplastic cells resemble classic HRS cells, but and adults with high proliferation index and no BCL2 rearrangement.
Blood 2012;120:2395–404.
morphological distinction from LP cells may be difficult in some 13. Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ:
cases. Thus, in the past, many cases were misdiagnosed as NLPHL. clinical implications and comparisons with partial involvement by
However, patients usually present with localized disease and tend follicular lymphoma. Blood 2011;118:2976–84.
to be older than patients with NLPHL. The genetic features are 14. Mamessier E, Song JY, Eberle FC, et al. Early lesions of follicular
similar to other variants of CHL. lymphoma: a genetic perspective. Haematologica 2014;99:481–8.
15. Karube K, Guo Y, Suzumiya J, et al. CD10-MUM1+ follicular lymphoma
lacks BCL2 gene translocation and shows characteristic biologic and
ON ThE hOrIZON clinical features. Blood 2007;109:3076–9.
16. Salaverria I, Philipp C, Oschlies I, et al. Translocations activating IRF4
• In recent years, there has been a greater appreciation of early events identify a subtype of germinal center-derived B-cell lymphoma affecting
in lymphoid neoplasia. predominantly children and young adults. Blood 2011;118:139–47.
• These early lesions in some ways can be considered equivalent to 17. Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14;18)-
benign neoplasms in the epithelial system. negative nodal follicular non-Hodgkin lymphoma characterized by a
• These are clonal proliferations of B or T cells that carry genetic aber- predominantly diffuse growth pattern and deletions in the chromosomal
rations associated with specific forms of lymphoid neoplasia: chronic region 1p36. Blood 2009;113:1053–61.
lymphocytic leukemia, multiple myeloma, follicular lymphoma, mantle 18. Loeffler M, Kreuz M, Haake A, et al. Genomic and epigenomic
cell lymphoma. co-evolution in follicular lymphomas. Leukemia 2015;29:456–63.
• Examples include monoclonal gammopathy of undermined significance, 19. Swerdlow SH. Pediatric follicular lymphomas, marginal zone lymphomas,
monoclonal B-lymphocytosis, in situ mantle cell neoplasia, in situ and marginal zone hyperplasia. Am J Clin Pathol 2004;122:S98–109.
follicular neoplasia, lymphomatoid papulosis, patch stage of mycosis 20. Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated
fungoides.
• Early lesions appear to lack the secondary and tertiary “hits” seen in lymphoid tissue lymphoma. J Clin Oncol 2005;23:6370–8.
lymphoid neoplasms that are clinically significant, and most patients 21. Watkins AJ, Huang Y, Ye H, et al. Splenic marginal zone lymphoma:
have a very low risk of clinical progression. characterization of 7q deletion and its value in diagnosis. J Pathol
• Current focus is to define the precise genetic features that distinguish 2010;220:461–74.
early lesions from lymphoma, assess the risk of clinical progression, 22. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling
and determine their clinical management. to predict survival after chemotherapy for diffuse large-B-cell lymphoma.
N Engl J Med 2002;346:1937–47.