Page 50 - Hybrid PBD 2022 Tg 4 - Kimia
P. 50
Kimia Tingkatan 4 Bab 2
PBD 2.4 Isotop dan Penggunaannya
PBD
PBD
Isotopes and Their Uses Buku Teks ms. 37 – 39
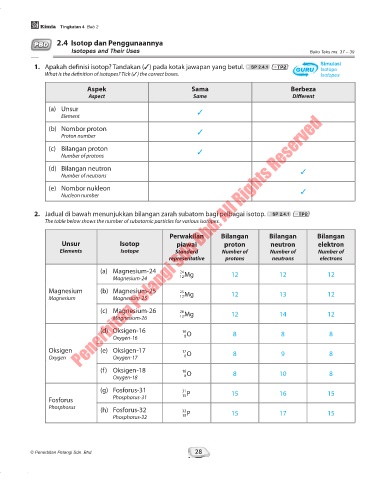
1. Apakah definisi isotop? Tandakan (3) pada kotak jawapan yang betul. SP 2.4.1 TP2 GURU Simulasi
Isotope
GURU
What is the definition of isotopes? Tick (3) the correct boxes. Isotopes
Aspek Sama Berbeza
Aspect Same Different
(a) Unsur 3
Penerbitan Pelangi Sdn Bhd. All Rights Reserved
Element
(b) Nombor proton 3
Proton number
(c) Bilangan proton 3
Number of protons
(d) Bilangan neutron 3
Number of neutrons
(e) Nombor nukleon 3
Nucleon number
2. Jadual di bawah menunjukkan bilangan zarah subatom bagi pelbagai isotop. SP 2.4.1 TP2
The table below shows the number of subatomic particles for various isotopes.
Perwakilan Bilangan Bilangan Bilangan
Unsur Isotop piawai proton neutron elektron
Elements Isotope Standard Number of Number of Number of
representative protons neutrons electrons
(a) Magnesium-24 24
Magnesium-24 12 Mg 12 12 12
Magnesium (b) Magnesium-25 25 Mg 12 13 12
Magnesium Magnesium-25 12
(c) Magnesium-26 26
Magnesium-26 12 Mg 12 14 12
(d) Oksigen-16 16 O 8 8 8
Oxygen-16 8
Oksigen (e) Oksigen-17 17
Oxygen Oxygen-17 8 O 8 9 8
(f) Oksigen-18 18 O 8 10 8
Oxygen-18 8
(g) Fosforus-31 31 P 15 16 15
Fosforus Phosphorus-31 15
Phosphorus (h) Fosforus-32 32
Phosphorus-32 15 P 15 17 15
© Penerbitan Pelangi Sdn. Bhd. 28
02 Bab 2.indd 28 10/29/21 5:13 PM