Page 69 - Hybrid PBD 2022 Tg 4 - Kimia
P. 69
Kimia Tingkatan 4 Bab 7
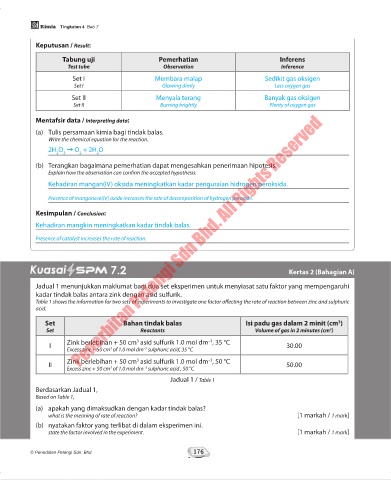
Keputusan / Result:
Tabung uji Pemerhatian Inferens
Test tube Observation Inference
Set I Membara malap Sedikit gas oksigen
Set I Glowing dimly Less oxygen gas
Set II Menyala terang Banyak gas oksigen
Set II Burning brightly Plenty of oxygen gas
Penerbitan Pelangi Sdn Bhd. All Rights Reserved
Mentafsir data / Interpreting data:
(a) Tulis persamaan kimia bagi tindak balas.
Write the chemical equation for the reaction.
2H O ➞ O + 2H O
2
2
2
2
(b) Terangkan bagaimana pemerhatian dapat mengesahkan penerimaan hipotesis.
Explain how the observation can confirm the accepted hypothesis.
Kehadiran mangan(IV) oksida meningkatkan kadar penguraian hidrogen peroksida.
Presence of manganese(IV) oxide increases the rate of decomposition of hydrogen peroxide.
Kesimpulan / Conclusion:
Kehadiran mangkin meningkatkan kadar tindak balas.
Presence of catalyst increases the rate of reaction.
7.2 Kertas 2 (Bahagian A)
Jadual 1 menunjukkan maklumat bagi dua set eksperimen untuk menyiasat satu faktor yang mempengaruhi
kadar tindak balas antara zink dengan asid sulfurik.
Table 1 shows the information for two sets of experiments to investigate one factor affecting the rate of reaction between zinc and sulphuric
acid.
Set Bahan tindak balas Isi padu gas dalam 2 minit (cm )
3
Set Reactants Volume of gas in 2 minutes (cm )
3
3
–3
I Zink berlebihan + 50 cm asid sulfurik 1.0 mol dm , 35 °C 30.00
3
–3
Excess zinc + 50 cm of 1.0 mol dm sulphuric acid, 35 °C
Zink berlebihan + 50 cm asid sulfurik 1.0 mol dm , 50 °C
–3
3
II 50.00
Excess zinc + 50 cm of 1.0 mol dm sulphuric acid , 50 °C
–3
3
Jadual 1 / Table 1
Berdasarkan Jadual 1,
Based on Table 1,
(a) apakah yang dimaksudkan dengan kadar tindak balas?
what is the meaning of rate of reaction? [1 markah / 1 mark]
(b) nyatakan faktor yang terlibat di dalam eksperimen ini.
state the factor involved in the experiment. [1 markah / 1 mark]
© Penerbitan Pelangi Sdn. Bhd. 176
07 Bab 7.indd 176 10/29/21 5:19 PM