Page 47 - T-I JOURNAL19-3
P. 47
ARGUS SYSTEM 607
Argus I was the first-generation epiretinal pros- NCT00407602). They ranged from 28 to 77 years old,
thesis approved for an investigational clinical trial and all had little to no light perception in both eyes.
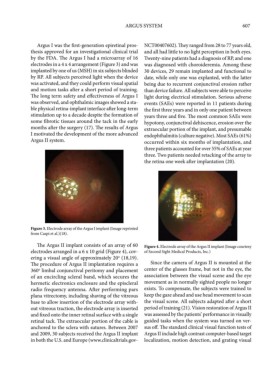
by the FDA. The Argus I had a microarray of 16 Twenty-nine patients had a diagnosis of RP, and one
electrodes in a 4 x 4 arrangement (Figure 3) and was was diagnosed with choroideremia. Among these
implanted by one of us (MSH) in six subjects blinded 30 devices, 29 remain implanted and functional to
by RP. All subjects perceived light when the device date, while only one was explanted, with the latter
was activated, and they could perform visual spatial being due to recurrent conjunctival erosion rather
and motion tasks after a short period of training. than device failure. All subjects were able to perceive
The long term safety and effectiveness of Argus I light during electrical stimulation. Serious adverse
was observed, and ophthalmic images showed a sta- events (SAEs) were reported in 11 patients during
ble physical retina-implant interface after long-term the first three years and in only one patient between
stimulation up to a decade despite the formation of years three and five. The most common SAEs were
some fibrotic tissues around the tack in the early hypotony, conjunctival dehiscence, erosion over the
months after the surgery (17). The results of Argus extraocular portion of the implant, and presumable
I motivated the development of the more advanced endophthalmitis (culture negative). Most SAEs (61%)
Argus II system. occurred within six months of implantation, and
three patients accounted for over 55% of SAEs at year
three. Two patients needed retacking of the array to
the retina one week after implantation (20).
Figure 3. Electrode array of the Argus I implant (Image reprinted
from Caspi et al.)(18).
The Argus II implant consists of an array of 60 Figure 4. Electrode array of the Argus II implant (Image courtesy
electrodes arranged in a 6 x 10 grid (Figure 4), cov- of Second Sight Medical Products, Inc.)
ering a visual angle of approximately 20 (18,19).
o
The procedure of Argus II implantation requires a Since the camera of Argus II is mounted at the
360 limbal conjunctival peritomy and placement center of the glasses frame, but not in the eye, the
o
of an encircling scleral band, which secures the association between the visual scene and the eye
hermetic electronics enclosure and the episcleral movement as in normally sighted people no longer
radio frequency antenna. After performing pars exists. To compensate, the subjects were trained to
plana vitrectomy, including shaving of the vitreous keep the gaze ahead and use head movement to scan
base to allow insertion of the electrode array with- the visual scene. All subjects adapted after a short
out vitreous traction, the electrode array is inserted period of training (21). Vision restoration of Argus II
and fixed onto the inner retinal surface with a single was assessed by the patients’ performance in visually
retinal tack. The extraocular portion of the cable is guided tasks when the system was turned on ver-
anchored to the sclera with sutures. Between 2007 sus off. The standard clinical visual function tests of
and 2009, 30 subjects received the Argus II implant Argus II include high contrast computer-based target
in both the U.S. and Europe (www.clinicaltrials.gov- localization, motion detection, and grating visual