Page 232 - ANUAL REPORT MOH 2017
P. 232
Capability in conducting local economic evaluation was strengthened in 2017 where more structured
trainings were conducted. As a result, more economic evaluation studies were successfully completed
as a component of technology assessment reports in 2017.
At the same time, MaHTAS continued to produce Health Technology Assessment (HTA), Technology
Review (mini-HTA) and Information Brief (rapid review) reports. In 2017, prioritisations of new HTA
topics for 2018-2019 were also conducted. Clinical Practice Guidelines (CPG) development and
implementation were continued in 2017 and emphasised on updating old CPGs.
In ensuring the quality and utilisation of reports and CPGs, MaHTAS continued to monitor and evaluate
its products through MaHTAS user feedbacks and relevant evaluation study. Health Technology
Assessment Impact study which officially started in 2016 was also continued.
ACHIEVEMENTS
Technology Assessment Reports and Clinical Practice Guidelines
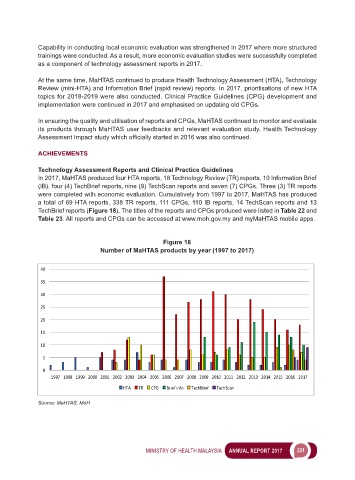
In 2017, MaHTAS produced four HTA reports, 18 Technology Review (TR) reports, 10 Information Brief
(IB), four (4) TechBrief reports, nine (9) TechScan reports and seven (7) CPGs. Three (3) TR reports
were completed with economic evaluation. Cumulatively from 1997 to 2017, MaHTAS has produced
a total of 69 HTA reports, 338 TR reports, 111 CPGs, 110 IB reports, 14 TechScan reports and 13
TechBrief reports (Figure 18). The titles of the reports and CPGs produced were listed in Table 22 and
Table 23. All reports and CPGs can be accessed at www.moh.gov.my and myMaHTAS mobile apps.
Figure 18
Number of MaHTAS products by year (1997 to 2017)
40
35
30
25
20
15
10
5
0
1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017
HTA TR CPG Brief info TechBrief TechScan
Source: MaHTAS, MoH
MINISTRY OF HEALTH MALAYSIA ANNUAL REPORT 2017 231