Page 75 - Supplement_Primary-1_Neat
P. 75
Week
I Can
CLASSIFYING MATTER 3/4
• Dolly Anne L. Idlisan
e identify the things we see by giving them names. Classifying
those that we identify makes it easier for us to make observations.
WAnd through our observation, we see their similarities and
differences.
Scientists are fond of classifying or grouping things because it makes
communicating and organizing information easy. Matter can be classified
based on their appearance (physical properties) and based on their
structure (chemical properties). One of the principal ways of classifying
matter is based on its simple observable state: solid, liquid, or gas.
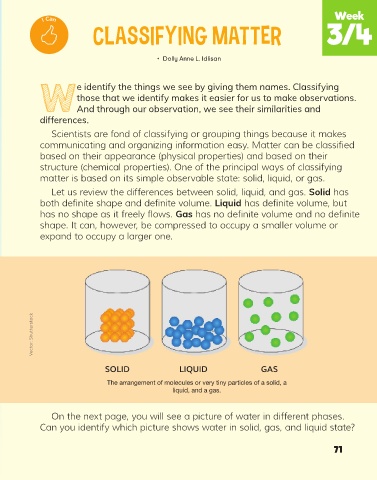
Let us review the differences between solid, liquid, and gas. Solid has
both definite shape and definite volume. Liquid has definite volume, but
has no shape as it freely flows. Gas has no definite volume and no definite
shape. It can, however, be compressed to occupy a smaller volume or
expand to occupy a larger one.
Vector: Shutterstock
SOLID LIQUID GAS
The arrangement of molecules or very tiny particles of a solid, a
liquid, and a gas.
On the next page, you will see a picture of water in different phases.
Can you identify which picture shows water in solid, gas, and liquid state?
71