Page 530 - SET 1 Koleksi Soalan dan Skema Jawapan SPM KBSM
P. 530
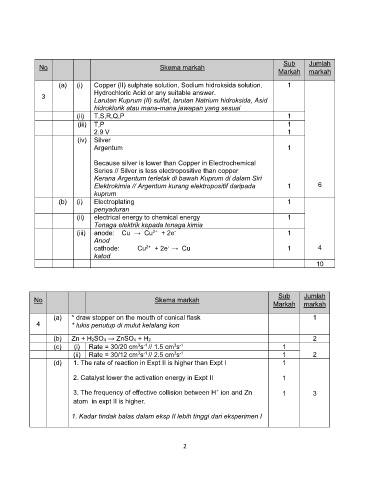
Sub Jumlah
No Skema markah
Markah markah
(a) (i) Copper (II) sulphate solution, Sodium hidroksida solution, 1
Hydrochloric Acid or any suitable answer.
3
Larutan Kuprum (II) sulfat, larutan Natrium hidroksida, Asid
hidroklorik atau mana-mana jawapan yang sesuai
(ii) T,S,R,Q,P 1
(iii) T,P 1
2.9 V 1
(iv) Silver
Argentum 1
Because silver is lower than Copper in Electrochemical
Series // Silver is less electropositive than copper
Kerana Argentum terletak di bawah Kuprum di dalam Siri
Elektrokimia // Argentum kurang elektropositif daripada 1 6
kuprum
(b) (i) Electroplating 1
penyaduran
(ii) electrical energy to chemical energy 1
Tenaga elektrik kepada tenaga kimia
(iii) 2+ + 2e - 1
Anod
-
2+
cathode: Cu + 2e 1 4
katod
10
Sub Jumlah
No Skema markah
Markah markah
(a) * draw stopper on the mouth of conical flask 1
4 * lukis penutup di mulut kelalang kon
(b) Zn + H2SO4 ZnSO4 + H2 2
3 -1
3 -1
(c) (i) Rate = 30/20 cm s // 1.5 cm s 1
3 -1
(ii) Rate = 30/12 cm s // 2.5 cm s 1 2
3 -1
(d) 1. The rate of reaction in Expt II is higher than Expt I 1
2. Catalyst lower the activation energy in Expt II 1
+
3. The frequency of effective collision between H ion and Zn 1 3
atom in expt II is higher.
1. Kadar tindak balas dalam eksp II lebih tinggi dari eksperimen I
2