Page 75 - Nilam_Publication_module_Chemistry_Form.pdf
P. 75
Chemistry Form 4 • MODULE
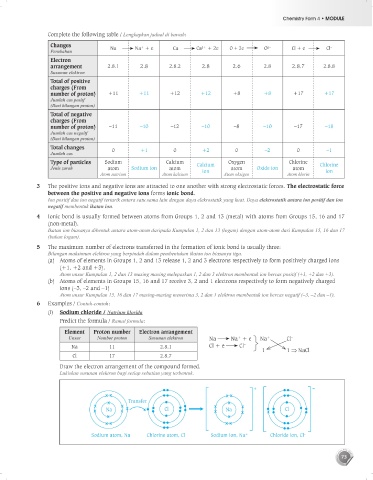
Complete the following table / Lengkapkan jadual di bawah:
Changes Na Na + e Ca Ca + 2e O + 2e O Cl + e Cl –
+
2–
2+
Perubahan
Electron
arrangement 2.8.1 2.8 2.8.2 2.8 2.6 2.8 2.8.7 2.8.8
Susunan elektron
Total of positive
charges (From
number of proton) +11 +11 +12 +12 +8 +8 +17 +17
Jumlah cas positf
(Dari bilangan proton)
Total of negative
charges (From
number of proton) –11 –10 –12 –10 –8 –10 –17 –18
Jumlah cas negaitf
(Dari bilangan proton)
Total changes 0 +1 0 +2 0 –2 0 –1
Jumlah cas
Type of particles Sodium Calcium Calcium Oxygen Chlorine Chlorine
Jenis zarah atom Sodium ion atom ion atom Oxide ion atom ion
Atom natrium Atom kalsium Atom oksigen Atom klorin
3 The positive ions and negative ions are attracted to one another with strong electrostatic forces. The electrostatic force
between the positive and negative ions forms ionic bond.
Ion positif dan ion negatif tertarik antara satu sama lain dengan daya elekrostatik yang kuat. Daya elektrostatik antara ion positif dan ion
negatif membentuk ikatan ion.
4 Ionic bond is usually formed between atoms from Groups 1, 2 and 13 (metal) with atoms from Groups 15, 16 and 17
(non-metal).
Ikatan ion biasanya dibentuk antara atom-atom daripada Kumpulan 1, 2 dan 13 (logam) dengan atom-atom dari Kumpulan 15, 16 dan 17
(bukan logam).
5 The maximum number of electrons transferred in the formation of ionic bond is usually three:
Bilangan maksimum elektron yang berpindah dalam pembentukan ikatan ion biasanya tiga.
(a) Atoms of elements in Groups 1, 2 and 13 release 1, 2 and 3 electrons respectively to form positively charged ions
(+1, +2 and +3).
Atom unsur Kumpulan 1, 2 dan 13 masing masing melepaskan 1, 2 dan 3 elektron membentuk ion bercas positif (+1, +2 dan +3).
(b) Atoms of elements in Groups 15, 16 and 17 receive 3, 2 and 1 electrons respectively to form negatively charged
ions (–3, –2 and –1)
Atom unsur Kumpulan 15, 16 dan 17 masing-masing menerima 3, 2 dan 1 elektron membentuk ion bercas negatif (–3, –2 dan –1).
6 Examples / Contoh-contoh:
(i) Sodium chloride / Natrium klorida
Predict the formula / Ramal formula:
Element Proton number Electron arrangement
+
Unsur Nombor proton Susunan elektron Na Na + e Na + Cl –
Na 11 2.8.1 Cl + e Cl –
1 1 NaCl
Cl 17 2.8.7
Draw the electron arrangement of the compound formed.
Lukiskan susunan elektron bagi setiap sebatian yang terbentuk.
Transfer
Pindah
Na
Na Na C1 Cl Na C1 Cl
Chloride ion, Cl
Sodium atom, Na
Sodium ion, Na
Atom natrium, Na Chlorine atom, Cl Ion natrium, Na + + Ion klorida, Cl – –
Atom klorin, Cl
73
Nilam Publication Sdn. Bhd.
04-Chem F4 (3P).indd 73 12/9/2011 5:57:09 PM