Page 136 - Science Class 6 Times Publication
P. 136
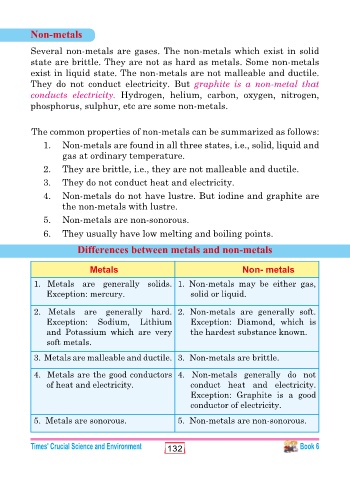
Non-metals
Several non-metals are gases. The non-metals which exist in solid
state are brittle. They are not as hard as metals. Some non-metals
exist in liquid state. The non-metals are not malleable and ductile.
They do not conduct electricity. But graphite is a non-metal that
conducts electricity. Hydrogen, helium, carbon, oxygen, nitrogen,
phosphorus, sulphur, etc are some non-metals.
The common properties of non-metals can be summarized as follows:
1. Non-metals are found in all three states, i.e., solid, liquid and
gas at ordinary temperature.
2. They are brittle, i.e., they are not malleable and ductile.
3. They do not conduct heat and electricity.
4. Non-metals do not have lustre. But iodine and graphite are
the non-metals with lustre.
5. Non-metals are non-sonorous.
6. They usually have low melting and boiling points.
Differences between metals and non-metals
Metals Non- metals
1. Metals are generally solids. 1. Non-metals may be either gas,
Exception: mercury. solid or liquid.
2. Metals are generally hard. 2. Non-metals are generally soft.
Exception: Sodium, Lithium Exception: Diamond, which is
and Potassium which are very the hardest substance known.
soft metals.
3. Metals are malleable and ductile. 3. Non-metals are brittle.
4. Metals are the good conductors 4. Non-metals generally do not
of heat and electricity. conduct heat and electricity.
Exception: Graphite is a good
conductor of electricity.
5. Metals are sonorous. 5. Non-metals are non-sonorous.
Times' Crucial Science and Environment 132 Book 6