Page 436 - fbkCardioDiabetes_2017
P. 436
412 Cardio Diabetes Medicine 2017
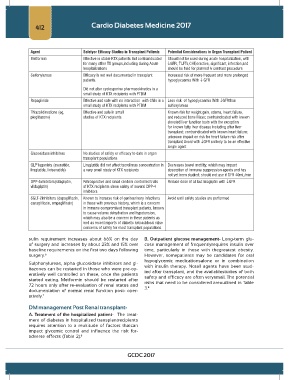
Agent Safetyor Efficacy Studies in Transplant Patients Potential Considerations in Organ Transplant Patient
Metformin Effective in stable KTX patients But contraindicated Should not be used during acute hospitalization, with
for many other TX groups,including during Acute ↓GFR, ↑LFTs,CHF,oractive, significant, infection;and
hospitalizations should be held for planned iv contrast procedure
Sulfonylureas Efficacy is not well documented in transplant Increased risk of more frequent and more prolonged
patients. hypoglycaemia With ↓ GFR
Did not alter cyclosporine pharmacokinetics in a
small study of KTX recipients with PTDM
Repaglinide Effective and safe with no interaction with CNIs in a Less risk of hypoglycaemia With ↓GFRthan
small study of KTX recipients with PTDM sulfonylureas
Thiazolidinedione (eg, Effective and safe in small Known risk for weight gain, edema, heart failure,
pioglitazone) studies of KTX recipients and reduced bone Mass; contraindicated with known
elevated liver function tests with the exception
for known fatty liver disease including after liver
transplant; contraindicated with known heart failure;
unknown impact on risk for heart failure risk after
transplant Avoid with ↓GFR unlikely to be an effective
single agent
Glucosidase inhibitors No studies of safety or efficacy to date in organ
transplant populations
GLP1agonists (exenatide, Liraglutide did not affect tacrolimus concentration in Decreases bowel motility, which may impact
liraglutide, lixisenatide) a very small study of KTX recipients absorption of immune suppression agents and has
not yet been studied; should not use if GFR 40mL/min
DPP-4inhibitors(sitagliptin, Retrospective and small random controlled trials Reduce dose of all but linagliptin with ↓GFR
vildagliptin) of KTX recipients show safety of several DPP-4
inhibitors
SGLT-2inhibitors (dapagliflozin, Known to increase risk of genitourinary infections Avoid until safety studies are performed
canagliflozin, empagliflozin) in those with previous history, which is a concern
in immune compromised transplant patients, known
to cause volume dehydration and hypotension,
which may also be a concern in these patients as
well as recent reports of diabetic ketoacidosis raise
concerns of safety for most transplant populations
sulin requirement increases about 66% on the day B. Outpatient glucose management- Long-term glu-
of surgery and increases by about 23% and 15% over cose management of frequentlyrequires insulin over
baseline requirements on the first two days following time, particularly in those with thegreatest obesity.
surgery. 6 However, somepatients may be candidates for oral
hypoglycemic medicationsalone or in combination
Sulphonylureas, alpha glucosidase inhibitors and gl-
itazones can be restarted in those who were pre-op- with insulin therapy. Notall agents have been stud-
eratively well controlled on these, once the patients ied after transplant, and the availablestudies of both
started eating. Metformin should be restarted after safety and efficacy are often verysmall. The potential
72 hours only after re-evaluation of renal status and risks that need to be considered areoutlined in Table
8
documentation of normal renal function post- oper- 3.
atively. 7
DM management Post Renal transplant-
A. Treatment of the hospitalized patient- The treat-
ment of diabetes in hospitalized transplantrecipients
requires attention to a multitude of factors thatcan
impact glycemic control and influence the risk for-
adverse effects (Table 2). 8
GCDC 2017