Page 487 - Clinical Application of Mechanical Ventilation
P. 487
Pharmacotherapy for Mechanical Ventilation 453
Inhalation of
A B
Obstructed Nitric Oxide
Airway
Normal
Airway
NO
Alveoli
NO
NO
© Cengage Learning 2014
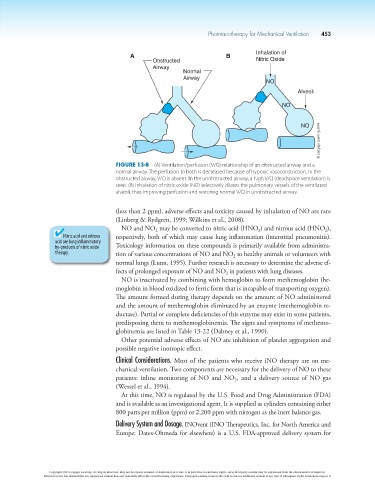
Figure 13-8 (A) Ventilation/perfusion (V/Q) relationship of an obstructed airway and a
normal airway. The perfusion to both is decreased because of hypoxic vasoconstriction. In the
obstructed airway, V/Q is absent. In the unobstructed airway, a high V/Q (deadspace ventilation) is
seen. (B) Inhalation of nitric oxide (NO) selectively dilates the pulmonary vessels of the ventilated
alveoli, thus improving perfusion and restoring normal V/Q in unobstructed airway.
(less than 2 ppm), adverse effects and toxicity caused by inhalation of NO are rare
(Linberg & Rydgren, 1999; Wilkins et al., 2008).
NO and NO may be converted to nitric acid (HNO ) and nitrous acid (HNO ),
2
3
2
Nitric acid and nitrous respectively, both of which may cause lung inflammation (interstitial pneumonitis).
acid are lung inflammatory
by-products of nitric oxide Toxicology information on these compounds is primarily available from administra-
therapy. tion of various concentrations of NO and NO to healthy animals or volunteers with
2
normal lungs (Lunn, 1995). Further research is necessary to determine the adverse ef-
fects of prolonged exposure of NO and NO in patients with lung diseases.
2
NO is inactivated by combining with hemoglobin to form methemoglobin (he-
moglobin in blood oxidized to ferric form that is incapable of transporting oxygen).
The amount formed during therapy depends on the amount of NO administered
and the amount of methemoglobin eliminated by an enzyme (methemoglobin re-
ductase). Partial or complete deficiencies of this enzyme may exist in some patients,
predisposing them to methemoglobinemia. The signs and symptoms of methemo-
globinemia are listed in Table 13-22 (Dabney et al., 1990).
Other potential adverse effects of NO are inhibition of platelet aggregation and
possible negative inotropic effect.
Clinical Considerations. Most of the patients who receive iNO therapy are on me-
chanical ventilation. Two components are necessary for the delivery of NO to these
patients: inline monitoring of NO and NO , and a delivery source of NO gas
2
(Wessel et al., 1994).
At this time, NO is regulated by the U.S. Food and Drug Administration (FDA)
and is available as an investigational agent. It is supplied as cylinders containing either
800 parts per million (ppm) or 2,200 ppm with nitrogen as the inert balance gas.
Delivery System and Dosage. INOvent (INO Therapeutics, Inc. for North America and
Europe; Datex-Ohmeda for elsewhere) is a U.S. FDA-approved delivery system for
Copyright 2013 Cengage Learning. All Rights Reserved. May not be copied, scanned, or duplicated, in whole or in part. Due to electronic rights, some third party content may be suppressed from the eBook and/or eChapter(s).
Editorial review has deemed that any suppressed content does not materially affect the overall learning experience. Cengage Learning reserves the right to remove additional content at any time if subsequent rights restrictions require it.