Page 861 - Cardiac Nursing
P. 861
LWBK340-c36_p823-841.qxd 29/06/2009 09:01 PM Page 837 Aptara
C HAP TE R 36 / Lipid Management and Cardiovascular Disease 837
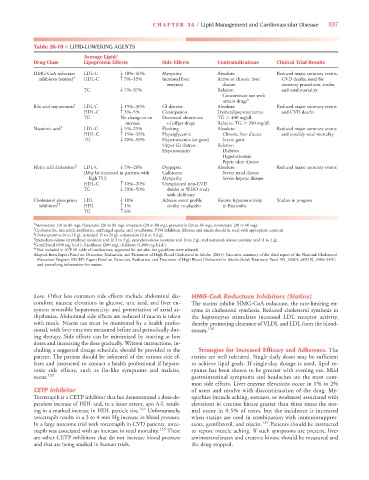
Table 36-10 ■ LIPID-LOWERING AGENTS
Average Lipid/
Drug Class Lipoprotein Effects Side Effects Contraindications Clinical Trial Results
HMG-CoA reductase LDL-C T 18%–60% Myopathy Absolute: Reduced major coronary events,
inhibitors (statins)* HDL-C c 5%–15% Increased liver Active or chronic liver CVD deaths, need for
enzymes disease coronary procedures, stroke,
TG T 7%–37% Relative: and total mortality
Concomitant use with
certain drugs †
Bile acid sequestrants ‡ LDL-C T 15%–30% GI distress Absolute: Reduced major coronary events
HDL-C c 3%–5% Constipation Dysbetalipoproteinemia and CVD deaths
TG No change or an Decreased absorption TG 400 mg/dL
increase of offger drugs Relative: TG 200 mg/dL
Nicotinic acid § LDL-C T 5%–25% Flushing Absolute: Reduced major coronary events
HDL-C c 15%–35% Hyperglycemia Chronic liver disease and possibly total mortality
TG T 20%–50% Hyperuricemia (or gout) Severe gout
Upper GI distress Relative:
Hepatotoxicity Diabetes
Hyperuricemia
Peptic ulcer disease
Fibric acid derivatives || LDL-C T 5%–20% Dyspepsia Absolute: Reduced major coronary events
(May be increased in patients with Gallstones Severe renal disease
high TG) Myopathy Severe hepatic disease
HDL-C c 10%–20% Unexplained non-CVD
TG T 20%–50% deaths in WHO study
with clofibrate
Cholesterol absorption LDL T 18% Adverse event profile Known hypersensitivity Studies in progress
inhibitors †† HDL c 1% similar to placebo to Ezetimibe
TG c 8%
*Atorvastatin (10 to 80 mg), fluvastatin (20 to 80 mg), lovastatin (20 to 80 mg), pravastatin (20 to 80 mg), simvastatin (20 to 80 mg).
† Cyclosporine, macrolide antibiotics, antifungal agents, and cytochrome P450 inhibitors (fibrates and niacin should be used with appropriate caution).
‡ Cholestyramine (4 to 16 g), colestipol (5 to 20 g), colesevelam (2.6 to 3.8 g).
§ Immediate-release (crystalline) nicotinic acid (1.5 to 3 g), extended-release nicotinic acid (1 to 2 g), and sustained-release nicotinic acid (1 to 2 g).
|| Gemfibrozil (600 mg b.i.d.), fenofibrate (200 mg), clofibrate (1,000 mg b.i.d.).
†† Not included in ATP III table of medications; approved for use after the guidelines were released.
Adapted from Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (2001). Executive summary of the third report of the National Cholesterol
Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA, 285(19), 2486–2497,
and prescribing information for statins.
dose. Other less common side effects include abdominal dis- HMG-CoA Reductase Inhibitors (Statins)
comfort; nausea; elevations in glucose, uric acid, and liver en- The statins inhibit HMG-CoA reductase, the rate-limiting en-
zymes; reversible hepatotoxicity; and potentiation of atrial ar- zyme in cholesterol synthesis. Reduced cholesterol synthesis in
rhythmias. Abdominal side effects are reduced if niacin is taken the hepatocytes stimulates increased LDL receptor activity,
with meals. Niacin use must be monitored by a health profes- thereby promoting clearance of VLDL and LDL from the blood-
sional, with liver enzymes measured before and periodically dur- stream. 136
ing therapy. Side effects can be minimized by starting at low
doses and increasing the dose gradually. Written instructions, in-
cluding a suggested dosage schedule, should be provided to the Strategies for Increased Efficacy and Adherence. The
patient. The patient should be informed of the various side ef- statins are well tolerated. Single daily doses may be sufficient
fects and instructed to contact a health professional if hepato- to achieve lipid goals. If single-day dosage is used, lipid re-
toxic side effects, such as flu-like symptoms and malaise, sponse has been shown to be greatest with evening use. Mild
occur. 133 gastrointestinal symptoms and headaches are the most com-
mon side effects. Liver enzyme elevations occur in 1% to 2%
CETP Inhibitor of users and resolve with discontinuation of the drug. My-
Torcetrapib is a CETP inhibitor that has demonstrated a dose-de- opathies (muscle aching, soreness, or weakness) associated with
pendent increase of HDL and, to a lesser extent, apo A-I, result- elevations in creatine kinase greater than three times the nor-
ing in a marked increase in HDL particle size. 134 Unfortunately, mal occur in 0.5% of users, but the incidence is increased
torcetrapib results in a 3 to 4 mm Hg increase in blood pressure. when statins are used in combination with immunosuppres-
In a large outcome trial with torcetrapib in CVD patients, torce- sants, gemfibrozil, and niacin. 137 Patients should be instructed
trapib was associated with an increase in total mortality. 135 There to report muscle aching. If such symptoms are present, liver
are other CETP-inhibitors that do not increase blood pressure aminotransferases and creatine kinase should be measured and
and that are being studied in human trials. the drug stopped.