Page 135 - APPENDIX B: Trials Investigating the Management of Acute Radiation-Induced Skin Reactions Reading Qualitative Research
P. 135
133
APPENDIX B: Trials Investigating the Management of Acute Radiation-Induced
Skin Reactions
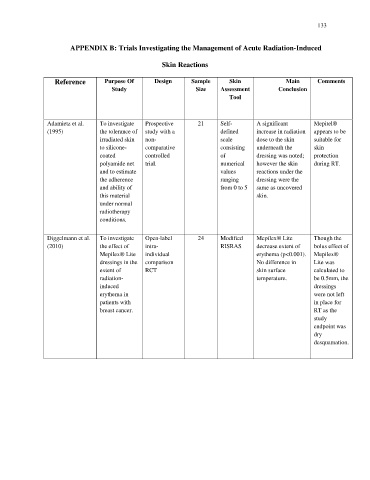
Reference Purpose Of Design Sample Skin Main Comments
Study Size Assessment Conclusion
Tool
Adamietz et al. To investigate Prospective 21 Self- A significant Mepitel®
(1995) the tolerance of study with a defined increase in radiation appears to be
irradiated skin non- scale dose to the skin suitable for
to silicone- comparative consisting underneath the skin
coated controlled of dressing was noted; protection
polyamide net trial. numerical however the skin during RT.
and to estimate values reactions under the
the adherence ranging dressing were the
and ability of from 0 to 5 same as uncovered
this material skin.
under normal
radiotherapy
conditions.
Diggelmann et al. To investigate Open-label 24 Modified Mepilex® Lite Though the
(2010) the effect of intra- RISRAS decrease extent of bolus effect of
Mepilex® Lite individual erythema (p<0.001). Mepilex®
dressings in the comparison No difference in Lite was
extent of RCT skin surface calculated to
radiation- temperature. be 0.5mm, the
induced dressings
erythema in were not left
patients with in place for
breast cancer. RT as the
study
endpoint was
dry
desquamation.