Page 1238 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1238
1084 Part VII Hematologic Malignancies
Homozygous
V617F mutation
9p
9pLOH
Hypothetical Heterozygous
mutation in V167F mutation
unknown in JAK2
gene “X” gene
Expansion of
9pLOH subclone
Clonal
regression
Normal
stem cell
Model B Model A
Clonal expansion
Onset of
myeloproliferative
disease 9pLOH-negative
cells
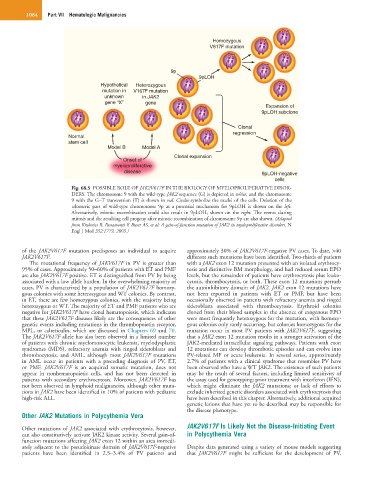
Fig. 68.5 POSSIBLE ROLE OF JAK2V617F IN THE BIOLOGY OF MYELOPROLIFERATIVE DISOR-
DERS. The chromosome 9 with the wild-type JAK2 sequence (G) is depicted in white, and the chromosome
9 with the G–T transversion (T) is shown in red. Circles symbolize the nuclei of the cells. Deletion of the
telomeric part of wild-type chromosome 9p as a potential mechanism for 9pLOH is shown on the left.
Alternatively, mitotic recombination could also result in 9pLOH, shown on the right. The events during
mitosis and the resulting cell progeny after mitotic recombination of chromosome 9p are also shown. (Adapted
from Kralovics R, Passamonti F, Buser AS, et al: A gain-of-function mutation of JAK2 in myeloproliferative disorders. N
Engl J Med 352:1779, 2005.)
of the JAK2V617F mutation predisposes an individual to acquire approximately 30% of JAK2V617F-negative PV cases. To date, >40
JAK2V617F. different such mutations have been identified. Two-thirds of patients
The mutational frequency of JAKV617F in PV is greater than with a JAK2 exon 12 mutation presented with an isolated erythrocy-
95% of cases. Approximately 50–60% of patients with ET and PMF tosis and distinctive BM morphology, and had reduced serum EPO
are also JAK2V617F positive. ET is distinguished from PV by being levels, but the remainder of patients have erythrocytosis plus leuko-
associated with a low allele burden. In the overwhelming majority of cytosis, thrombocytosis, or both. These exon 12 mutations perturb
cases, PV is characterized by a population of JAK2V617F homozy- the autoinhibitory domain of JAK2. JAK2 exon 12 mutations have
gous colonies with some heterozygous and WT colonies. By contrast, not been reported in patients with ET or PMF, but have been
in ET, there are few homozygous colonies, with the majority being occasionally observed in patients with refractory anemia and ringed
heterozygous or WT. The majority of ET and PMF patients who are sideroblasts associated with thrombocytosis. Erythroid colonies
negative for JAK2V617F have clonal hematopoiesis, which indicates cloned from their blood samples in the absence of exogenous EPO
that these JAK2V617F diseases likely are the consequences of other were most frequently heterozygous for the mutation, with homozy-
genetic events including mutations in the thrombopoietin receptor, gous colonies only rarely occurring, but colonies homozygous for the
MPL, or calreticulin, which are discussed in Chapters 69 and 70. mutation occur in most PV patients with JAK2V617F, suggesting
The JAK2V617F allele has also been observed in a limited number that a JAK2 exon 12 mutation results in a stronger activation of the
of patients with chronic myelomonocytic leukemia, myelodysplastic JAK2-mediated intracellular signaling pathways. Patients with exon
syndromes (MDS), refractory anemia with ringed sideroblasts and 12 mutations can develop thrombotic episodes and can evolve into
thrombocytosis, and AML, although most JAK2V617F mutations PV-related MF or acute leukemia. In several series, approximately
in AML occur in patients with a preceding diagnosis of PV, ET, 2.7% of patients with a clinical syndrome that resembles PV have
or PMF. JAK2V617F is an acquired somatic mutation, does not been observed who have a WT JAK2. The existence of such patients
appear in nonhematopoietic cells, and has not been detected in may be the result of several factors, including limited sensitivity of
patients with secondary erythrocytosis. Moreover, JAK2V617F has the assay used for genotyping; prior treatment with interferon (IFN),
not been observed in lymphoid malignancies, although other muta- which might eliminate the JAK2 mutations; or lack of efforts to
tions in JAK2 have been identified in 10% of patients with pediatric exclude inherited genetic disorders associated with erythrocytosis that
high-risk ALL. have been described in this chapter. Alternatively, additional acquired
genetic lesions that have yet to be described may be responsible for
the disease phenotype.
Other JAK2 Mutations in Polycythemia Vera
JAK2V617F Is Likely Not the Disease-Initiating Event
Other mutations of JAK2 associated with erythrocytosis, however,
can also constitutively activate JAK2 kinase activity. Several gain-of- in Polycythemia Vera
function mutations affecting JAK2 exon 12 within an area immedi-
ately adjacent to the pseudokinase domain of JAK2V617F-negative Despite data generated using a variety of mouse models suggesting
patients have been identified in 2.5–3.4% of PV patients and that JAK2V617F might be sufficient for the development of PV,