Page 1718 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1718
1528 Part IX Cell-Based Therapies
donor transplantation found that recipients of higher marrow cell cells and time to platelet engraftment than found with the overall
+
doses experienced faster neutrophil and platelet engraftment, as well number of CD34 cells. Coexpression of adhesion molecules is also
107
as better leukemia-free survival. Pretreatment of the marrow donor predictive of hematopoietic recovery, with the same pathways impor-
112
with either GM-CSF or G-CSF may increase the number of myeloid tant for both mobilization and homing of HSCs. Given the limited
+
progenitor cells harvested and decrease the period of posttransplant range in recovery times when adequate numbers of CD34 cells are
+
aplasia to that achievable by PBSC transplantation. CD34 cell dose collected and infused, however, this additional information is cur-
is now being correlated with transplant outcomes—with more rapid rently of limited clinical value.
+
engraftment kinetics, possibly lower transplant-related mortality, and CD34 cell dose is also predictive of outcome of allogeneic PBSC
+
better overall survival, for example, in recipients of allogeneic bone (and marrow) transplantation. 108,113 Higher CD34 cell doses result
+
108
marrow products containing higher quantities of CD34 cells. This in better neutrophil and platelet engraftments after either related or
assay should be a routine component of bone marrow product quality unrelated donor transplantation and may correlate with better sur-
control. 108 vival after bone marrow transplantation, but may also be associated
with the development of more chronic GVHD.
Definition of Adequate PBSC Component(s)
Tumor Cell Contamination
+
The quantity of CD34 cells in a PBSC component varies greatly and
is dependent on the number in the peripheral blood at the time of The probability that tumor cell contamination of HSC product could
114
apheresis, the volume of blood processed, and the efficiency of the contribute to relapse was demonstrated by Brenner and colleagues
apheresis device. Therefore, any definition of an adequate component in studies involving the autologous transplantation of genetically
+
cannot include a set number of CD34 cells to be contained in any marked marrow cells, as well as in individual case reports describing
single apheresis component. Instead, one or more components will the transmission of malignancy to allogeneic recipients from donors
be collected to meet the appropriate dose of these cells for transplan- with occult disease at time of harvesting. 27,28 Sensitive immunocyto-
+
tation. The dose of CD34 cells required for infusion depends on the staining techniques, clonal assays, flow cytometric analysis, and
intended treatment regimen. For marrow-ablative regimens, increas- polymerase chain reaction amplification of malignant genetic mate-
+
ingly higher CD34 doses results in greater likelihood of rapid rial detect tumor cells in the autologous PBSC components of many
+
115
109
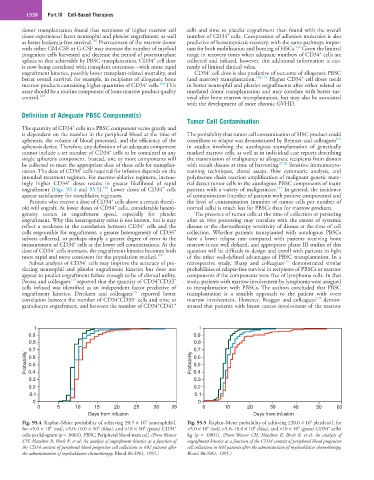
engraftment (Figs. 95.4 and 95.5). Lower doses of CD34 cells patients with a variety of malignancies. In general, the incidence
appear satisfactory for nonablative regimens. of contamination (number of patients with positive components) and
+
Patients who receive a dose of CD34 cells above a certain thresh- the level of contamination (number of tumor cells per number of
+
old will engraft. At lower doses of CD34 cells, considerable hetero- normal cells) is much less for PBSCs than for marrow products.
geneity occurs in engraftment speed, especially for platelet The presence of tumor cells at the time of collection or persisting
engraftment. Why this heterogeneity exists is not known, but it may after ex vivo processing may correlate with the extent of systemic
+
reflect a weakness in the correlation between CD34 cells and the disease or the chemotherapy sensitivity of disease at the time of cell
+
cells responsible for engraftment, a greater heterogeneity of CD34 collection. Whether patients transplanted with autologous PBSCs
subsets collected, or perhaps simply a greater degree of error in the have a lower relapse rate compared with patients receiving bone
+
measurement of CD34 cells at the lower cell concentrations. As the marrow is not well defined, and appropriate phase III studies of this
+
dose of CD34 cells increases, the engraftment kinetics becomes both question will be difficult to design and enroll with patients in light
more rapid and more consistent for the population studied. 109 of the other well-defined advantages of PBSC transplantation. In a
+
116
Subset analysis of CD34 cells may improve the accuracy of pre- retrospective study, Sharp and colleagues demonstrated similar
dicting neutrophil and platelet engraftment kinetics but does not probabilities of relapse-free survival in recipients of PBSCs or marrow
appear to predict engraftment failure enough to be of clinical utility. components if the components were free of lymphoma cells. In that
−
+
110
Pecora and colleagues reported that the quantity of CD34 CD33 study, patients with marrow involvement by lymphoma were assigned
cells infused was identified as an independent factor predictive of to transplantation with PBSCs. The authors concluded that PBSC
111
engraftment kinetics. Dercksen and colleagues reported better transplantation is a sensible approach to the patient with overt
−
+
117
correlation between the number of CD34 CD33 cells and time to marrow involvement. However, Brugger and colleagues demon-
+
+
granulocyte engraftment, and between the number of CD34 CD41 strated that patients with breast cancer involvement of the marrow
1 1
0.9 0.9
0.8 0.8
0.7 0.7
Probability 0.6 Probability 0.6
0.5
0.5
0.4
0.3
0.3 0.4
0.2 0.2
0.1 0.1
0 0
0 5 10 15 20 25 30 35 0 10 20 30 40 50 60
Days from infusion Days from infusion
9
9
Fig. 95.4 Kaplan–Meier probability of achieving ≥0.5 × 10 neutrophils/L Fig. 95.5 Kaplan–Meier probability of achieving ≥20.0 × 10 platelets/L for
+
+
6
6
6
6
6
6
for <5.0 × 10 (red), >5.0–10.0 × 10 (blue), and >10 × 10 (green) CD34 <5.0 × 10 (red), >5.0–10.0 × 10 (blue), and >10 × 10 (green) CD34 cells/
cells per kilogram (p = .0001). PBSC, Peripheral blood stem cell. (From Weaver kg (p = .0001). (From Weaver CH, Hazelton B, Birch R, et al: An analysis of
CH, Hazelton B, Birch R, et al: An analysis of engraftment kinetics as a function of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor
the CD34 content of peripheral blood progenitor cell collections in 692 patients after cell collections in 692 patients after the administration of myeloablative chemotherapy.
the administration of myeloablative chemotherapy. Blood 86:3961, 1995.) Blood 86:3961, 1995.)