Page 1824 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1824
1626 Part X Transplantation
T Cell Activation T Cell Proliferation Proliferating
ALLOREACTIVE
Peptide-MHC CD80/CD86 Receptor Cy day 3 cells are killed
CD28 TCR
T cell
Alloreactive
T cells CD40 CD40L IL-2 Activated
effector
Dendritic cell T cell
Anti-CMV
Anti-CMV
Non-alloreactive
T cells
Anti-HSV
Anti-HSV
Non-proliferating
non-alloreactive
cells are spared
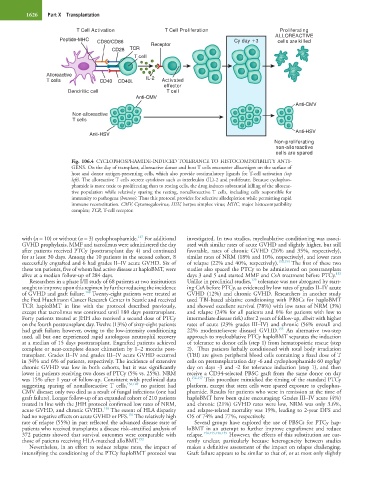
Fig. 106.4 CYCLOPHOSPHAMIDE-INDUCED TOLERANCE TO HISTOCOMPATIBILITY ANTI-
GENS. On the day of transplant, alloreactive donor and host T cells encounter alloantigen on the surface of
host and donor antigen-presenting cells, which also provide costimulatory ligands for T-cell activation (top
left). The alloreactive T cells secrete cytokines such as interleukin (IL)-2 and proliferate. Because cyclophos-
phamide is more toxic to proliferating than to resting cells, the drug induces substantial killing of the alloreac-
tive population while relatively sparing the resting, nonalloreactive T cells, including cells responsible for
immunity to pathogens (bottom). Thus this protocol provides for selective allodepletion while permitting rapid
immune reconstitution. CMV, Cytomegalovirus; HSV, herpes simplex virus; MHC, major histocompatibility
complex; TCR, T-cell receptor.
147
with (n = 10) or without (n = 3) cyclophosphamide. For additional investigated. In two studies, myeloablative conditioning was associ-
GVHD prophylaxis, MMF and tacrolimus were administered the day ated with similar rates of acute GVHD and slightly higher, but still
after patients received PTCy (posttransplant day 4) and continued favorable, rates of chronic GVHD (26% and 35%, respectively),
for at least 30 days. Among the 10 patients in the second cohort, 8 similar rates of NRM (18% and 10%, respectively), and lower rates
successfully engrafted and 6 had grades II–IV acute GVHD. Six of of relapse (22% and 40%, respectively). 153,154 The first of these two
these ten patients, five of whom had active disease at haploBMT, were studies also spaced the PTCy to be administered on posttransplant
153
alive at a median follow-up of 284 days. days 3 and 5 and started MMF and CsA treatment before PTCy.
129
Researchers in a phase I/II study of 68 patients at two institutions Unlike in preclinical studies, tolerance was not abrogated by start-
sought to improve upon this regimen by further reducing the incidence ing CsA before PTCy, as evidenced by low rates of grades II–IV acute
148
of GVHD and graft failure. Twenty-eight patients were treated at GVHD (12%) and chronic GVHD. Researchers in another study
the Fred Hutchinson Cancer Research Center in Seattle and received used TBI-based ablative conditioning with PBSCs for haploBMT
TCR haploBMT in line with the protocol described previously, and showed excellent survival (78%) with low rates of NRM (3%)
except that tacrolimus was continued until 180 days posttransplant. and relapse (24% for all patients and 0% for patients with low to
Forty patients treated at JHH also received a second dose of PTCy intermediate disease risk) after 2 years of follow-up, albeit with higher
on the fourth posttransplant day. Twelve (13%) of sixty-eight patients rates of acute (23% grades III–IV) and chronic (56% overall and
155
had graft failure; however, owing to the low-intensity conditioning 22% moderate/severe disease) GVHD. An alternative two-step
used, all but one experienced rapid autologous neutrophil recovery approach to myeloablative PTCy haploBMT separates the induction
at a median of 15 days posttransplant. Engrafted patients achieved of tolerance to donor cells (step 1) from hematopoietic rescue (step
complete or near-complete donor chimerism by 1–2 months post- 2). Thus patients lethally conditioned with total body irradiation
transplant. Grades II–IV and grades III–IV acute GVHD occurred (TBI) are given peripheral blood cells containing a fixed dose of T
in 34% and 6% of patients, respectively. The incidence of extensive cells on pretransplantation day -6 and cyclophosphamide 60 mg/kg/
chronic GVHD was low in both cohorts, but it was significantly day on days -3 and -2 for tolerance induction (step 1), and then
lower in patients receiving two doses of PTCy (5% vs. 25%). NRM receive a CD34-selected PBSC graft from the same donor on day
was 15% after 1 year of follow-up. Consistent with preclinical data 0. 156,157 This procedure mimicked the timing of the standard PTCy
suggesting sparing of nonalloreactive T cells, 136,149 no patient had platform, except that stem cells were spared exposure to cyclophos-
CMV disease; only two died as a result of fungal infections (one had phamide. Results for patients who were in remission at the time of
graft failure). Longer follow-up of an expanded cohort of 210 patients haploBMT have been quite encouraging: Grades III–IV acute (4%)
treated in line with the JHH protocol confirmed low rates of NRM, and chronic (21%) GVHD rates were low, NRM was only 3.6%,
150
acute GVHD, and chronic GVHD. The extent of HLA disparity and relapse-related mortality was 19%, leading to 2-year DFS and
151
had no negative effects on acute GVHD or PFS. The relatively high OS of 74% and 77%, respectively.
rate of relapse (55%) in part reflected the advanced disease state of Several groups have explored the use of PBSCs for PTCy hap-
patients who received transplants; a disease risk–stratified analysis of loBMT in an attempt to further improve engraftment and reduce
372 patients showed that survival outcomes were comparable with relapse. 154,155,158,159 However, the effects of this substitution are cur-
those of patients receiving HLA-matched alloBMT. 152 rently unclear, particularly because heterogeneity between studies
Nevertheless, in an effort to reduce relapse rates, the impact of makes a definitive assessment of the impact on relapse challenging.
intensifying the conditioning of the PTCy haploBMT protocol was Graft failure appears to be similar to that of, or at most only slightly