Page 287 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 287
238 Part III Immunologic Basis of Hematology
incorporation of an additional intracellular signaling domain derived
from costimulatory molecules, such as CD28 or 4-1BB, into second-
generation CARs augmented CAR-T-cell activation and antitumor
Antigen efficacy, and third-generation CARs include signaling domains from
recognition two costimulatory receptors. Currently, most CARs adhere to the
second-generation model. Which intracellular costimulatory domains
will work best in CARs and how many costimulatory domains are
required for optimal T-cell activation and antitumor efficacy are
under active investigation. Other outstanding questions in the bio-
Transmembrane chemistry of CARs concern the optimal number of functional ITAMs
domain present in ζ chain domains and the length and composition of the
transmembrane hinge domain and interdomain junctions. Defining
the biochemistry and signal transduction of CARs should permit
Co-stimulatory broadening its use to more common malignancies.
domain
The examples presented here are only a small subset of novel
approaches in use or being tested to modulate immune cell function
based upon our understanding of the molecular basis of T-cell activa-
CD3 chain tion. It is anticipated that as more is learned about the molecules and
pathways critical for control of T cell–mediated immunity, additional
new agents with greater efficacy and improved safety profiles will
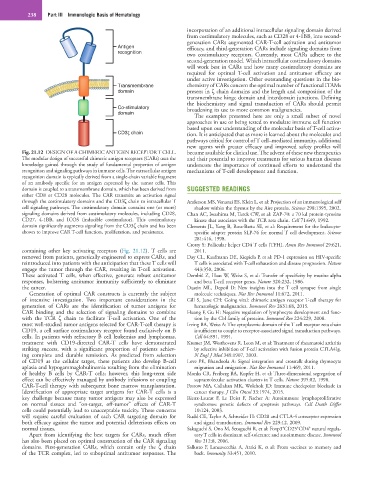
Fig. 21.12 DESIGN OF A CHIMERIC ANTIGEN RECEPTOR T CELL. become available for clinical use. The advent of these new therapeutics
The modular design of successful chimeric antigen receptors (CARs) uses the and their potential to improve treatments for serious human diseases
knowledge gained through the study of fundamental properties of antigen underscore the importance of continued efforts to understand the
recognition and signaling pathways in immune cells. The extracellular antigen mechanisms of T-cell development and function.
recognition domain is typically derived from a single-chain variable fragment
of an antibody specific for an antigen expressed by the tumor cells. This
domain is coupled to a transmembrane domain, which has been derived from SUGGESTED READINGS
either CD8 or CD28 molecules. The CAR transmits an activation signal
through the costimulatory domains and the CD3ζ chain to intracellular T Anderson MS, Venanzi ES, Klein L, et al: Projection of an immunological self
cell signaling pathways. The costimulatory domain contains one (or more) shadow within the thymus by the Aire protein. Science 298:1395, 2002.
signaling domains derived from costimulatory molecules, including CD28, Chan AC, Iwashima M, Turck CW, et al: ZAP-70: a 70 kd protein-tyrosine
CD27, 4-1BB, and ICOS (inducible costimulator). This costimulatory kinase that associates with the TCR zeta chain. Cell 71:649, 1992.
domain significantly augments signaling from the CD3ζ chain and has been Clements JL, Yang B, Ross-Barta SE, et al: Requirement for the leukocyte-
shown to improve CAR T-cell function, proliferation, and persistence. specific adapter protein SLP-76 for normal T cell development. Science
281:416, 1998.
Crotty S: Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29:621,
containing other key activating receptors (Fig. 21.12). T cells are 2011.
removed from patients, genetically engineered to express CARs, and Day CL, Kaufmann DE, Kiepiela P, et al: PD-1 expression on HIV-specific
reintroduced into patients with the anticipation that these T cells will T cells is associated with T-cell exhaustion and disease progression. Nature
engage the tumor through the CAR, resulting in T-cell activation. 443:350, 2006.
These activated T cells, when effective, generate robust antitumor Dembić Z, Haas W, Weiss S, et al: Transfer of specificity by murine alpha
responses, bolstering antitumor immunity sufficiently to eliminate and beta T-cell receptor genes. Nature 320:232, 1986.
the cancer. Dustin ML, Depoil D: New insights into the T cell synapse from single
Generation of optimal CAR constructs is currently the subject molecule techniques. Nat Rev Immunol 11:672, 2011.
of intensive investigation. Two important considerations in the Gill S, June CH: Going viral: chimeric antigen receptor T-cell therapy for
generation of CARs are the identification of tumor antigens for hematologic malignancies. Immunol Rev 2631:68, 2015.
CAR binding and the selection of signaling domains to combine Huang F, Gu H: Negative regulation of lymphocyte development and func-
with the TCR ζ chain to facilitate T-cell activation. One of the tion by the Cbl family of proteins. Immunol Rev 224:229, 2008.
most well-studied tumor antigens selected for CAR-T-cell therapy is Irving BA, Weiss A: The cytoplasmic domain of the T cell receptor zeta chain
CD19, a cell surface costimulatory receptor found exclusively on B is sufficient to couple to receptor-associated signal transduction pathways.
cells. In patients with refractory B cell leukemias and lymphomas, Cell 64:891, 1991.
treatment with CD19-directed CAR-T cells have demonstrated Kremer JM, Westhovens R, Leon M, et al: Treatment of rheumatoid arthritis
striking success, with a significant proportion of patients achiev- by selective inhibition of T-cell activation with fusion protein CTLA4Ig.
ing complete and durable remission. As predicted from selection N Engl J Med 349:1907, 2003.
of CD19 as the cellular target, these patients also develop B-cell Love PE, Bhandoola A: Signal integration and crosstalk during thymocyte
aplasia and hypogammaglobulinemia resulting from the elimination migration and emigration. Nat Rev Immunol 11:469, 2011.
of healthy B cells by CAR-T cells; however, this long-term side Monks CR, Freiberg BA, Kupfer H, et al: Three-dimensional segregation of
effect can be effectively managed by antibody infusions or coupling supramolecular activation clusters in T cells. Nature 395:82, 1998.
CAR-T-cell therapy with subsequent bone marrow transplantation. Postow MA, Callahan MK, Wolchok JD: Immune checkpoint blockade in
Identification of appropriate target antigens for CAR-T cells is a cancer therapy. J Clin Oncol 33:1974, 2015.
key challenge because many tumor antigens may also be expressed Rieux-Laucat F, Le Deist F, Fischer A: Autoimmune lymphoproliferative
on normal tissues and “on-target, off-tumor” effects of CAR-T syndromes: genetic defects of apoptosis pathways. Cell Death Differ
cells could potentially lead to unacceptable toxicity. These concerns 10:124, 2003.
will require careful evaluation of each CAR targeting domain for Rudd CE, Taylor A, Schneider H: CD28 and CTLA-4 coreceptor expression
both efficacy against the tumor and potential deleterious effects on and signal transduction. Immunol Rev 229:12, 2009.
+
+
+
normal tissues. Sakaguchi S, Ono M, Setoguchi R, et al: Foxp3 CD25 CD4 natural regula-
Apart from identifying the best targets for CARs, much effort tory T cells in dominant self-tolerance and autoimmune disease. Immunol
has also been placed on optimal construction of the CAR signaling Rev 212:8, 2006.
domains. First-generation CARs, which contain only the ζ chain Sallusto F, Lanzavecchia A, Araki K, et al: From vaccines to memory and
of the TCR complex, led to suboptimal antitumor responses. The back. Immunity 33:451, 2010.