Page 293 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 293
244 Part III Immunologic Basis of Hematology
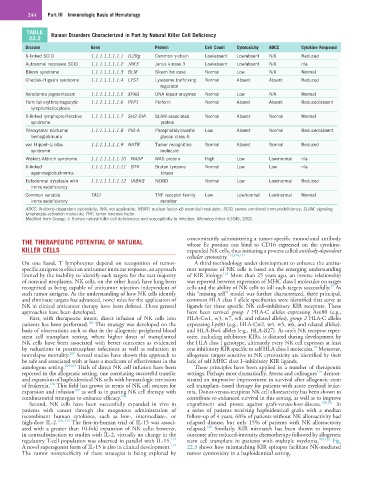
TABLE Human Disorders Characterized in Part by Natural Killer Cell Deficiency
22.2
Disease Gene Protein Cell Count Cytotoxicity ADCC Cytokine Response
X-linked SCID 1.1.1.1.1.1.1.1 IL2Rg Common γ-chain Low/absent Low/absent N/A Reduced
Autosomal recessive SCID 1.1.1.1.1.1.1.2 JAK3 Janus kinase 3 Low/absent Low/absent N/A n/a
Bloom syndrome 1.1.1.1.1.1.1.3 BLM Bloom helicase Normal Low N/A Normal
Chediak-Higashi syndrome 1.1.1.1.1.1.1.4 LYST Lysosome trafficking Normal Absent Absent Reduced
regulator
Xeroderma pigmentosum 1.1.1.1.1.1.1.5 XPAG DNA repair enzymes Normal Low N/A Normal
Familial erythrophagocytic 1.1.1.1.1.1.1.6 PFP1 Perforin Normal Absent Absent Reduced/absent
lymphohistiocytosis
X-linked lymphoproliferative 1.1.1.1.1.1.1.7 SH2-DIA SLAM-associated Normal Absent Normal Normal
syndrome protein
Paroxysmal nocturnal 1.1.1.1.1.1.1.8 PIG-A Phosphatidylinositol Low Absent Normal Reduced/absent
hemoglobinuria glycan class A
von Hippel–Lindau 1.1.1.1.1.1.1.9 NKTR Tumor recognition Normal Absent Normal Reduced
syndrome molecule
Wiskott-Aldrich syndrome 1.1.1.1.1.1.1.10 WASP WAS protein High Low Low/normal n/a
X-linked 1.1.1.1.1.1.1.11 BTK Bruton tyrosine Normal Low Low n/a
agammaglobulinemia kinase
Ectodermal dysplasia with 1.1.1.1.1.1.1.12 IKBKG NEMO Normal Low Low/normal Reduced
immunodeficiency
Common variable TACI TNF receptor family Low Low/normal Low/normal Normal
immunodeficiency member
ADCC, Antibody-dependent cytotoxicity; N/A, not applicable; NEMO, nuclear factor-κB essential modulator; SCID, severe combined immunodeficiency; SLAM, signaling
lymphocyte-activation molecule; TNF, tumor necrosis factor.
Modified from Orange J: Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect 4:1545, 2002.
THE THERAPEUTIC POTENTIAL OF NATURAL concomitantly administering a tumor-specific monoclonal antibody
whose Fc portion can bind to CD16 expressed on the cytokine-
KILLER CELLS expanded NK cells, thus initiating a process called antibody-dependent
cellular cytotoxicty. 112,116,117
On one hand, T lymphocytes depend on recognition of tumor- A third methodology under development to enhance the antitu-
specific antigens to effect an antitumor immune response, an approach mor response of NK cells is based on the emerging understanding
118
limited by the inability to identify such targets for the vast majority of KIR biology. More than 25 years ago, an inverse relationship
of nonviral neoplasms. NK cells, on the other hand, have long been was reported between expression of MHC class I molecules on target
39
recognized as being capable of antitumor rejection independent of cells and the ability of NK cells to kill such targets successfully. As
such tumor antigens. As the understanding of how NK cells identify this “missing self” model was further characterized, three principal,
and eliminate targets has advanced, novel roles for the application of common HLA class I allele specificities were identified that serve as
NK in clinical anticancer therapy have been defined. Three general ligands for three specific NK cell–inhibitory KIR receptors. These
approaches have been developed. have been termed group 1 HLA-C alleles expressing Asn80 (e.g.,
First, with therapeutic intent, direct infusion of NK cells into HLA-Cw1, w3, w7, w8, and related alleles), group 2 HLA-C alleles
102
patients has been performed. This strategy was developed on the expressing Lys80 (e.g., HLA-Cw2, w4, w5, w6, and related alleles),
basis of observations such as that in the allogeneic peripheral blood and HLA-Bw4 alleles (e.g., HLA-B27). As one’s NK receptor reper-
stem cell transplant setting, where higher doses of transplanted toire, including inhibitory KIRs, is dictated during development by
NK cells have been associated with better outcomes as evidenced the HLA class I genotype, ultimately every NK cell expresses at least
18
by reductions in posttransplant infections as well as reduction in one inhibitory KIR specific to self HLA class I molecules. Moreover,
103
nonrelapse mortality. Several studies have shown this approach to allogeneic targets sensitive to NK cytotoxicity are identified by their
be safe and associated with at least a modicum of effectiveness in the lack of self MHC class I–inhibitory KIR ligands.
autologous setting. 104,105 Trials of direct NK cell infusion have been These principles have been applied in a number of therapeutic
119
reported in the allogeneic setting, one correlating successful transfer settings. Perhaps most dramatically, Aversa and colleagues demon-
and expansion of haploidentical NK cells with hematologic remission strated an impressive improvement in survival after allogeneic stem
106
of leukemia. This field has grown in terms of NK cell sources for cell transplant–based therapy for patients with acute myeloid leuke-
107
expansion and infusion as well as in pairing NK cell therapy with mia. Donor-versus-recipient NK cell alloreactivity has been shown to
combinatorial strategies to enhance efficacy. 108 contribute to enhanced survival in this setting, as well as to improve
Second, NK cells have been successfully expanded in vivo in engraftment and protec against graft-versus-host-disease. 120,121 In
patients with cancer through the exogenous administration of a series of patients receiving haploidentical grafts with a median
recombinant human cytokines, such as low-, intermediate-, or follow-up of 4 years, 68% of patients without NK alloreactivity had
high-dose IL-2. 109–113 The first-in-human trial of IL-15 was associ- relapsed disease, but only 15% of patients with NK alloreactivity
120
ated with a greater than 10-fold expansion of NK cells; however, relapsed. Similarly, KIR mismatch has been shown to improve
in contradistinction to studies with IL-2, virtually no change in the outcome after reduced-intensity chemotherapy followed by allogeneic
114
regulatory T-cell population was observed in parallel with IL-15. stem cell transplant in patients with multiple myeloma. 109,122 Fig.
115
A novel superagonist form of IL-15 is also in clinical development. 22.3 shows how mismatching KIR epitopes facilitate NK-mediated
The tumor nonspecificity of these strategies is being explored by tumor cytotoxicity in a haploidentical setting.