Page 294 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 294
Chapter 22 Natural Killer Cell Immunity 245
Donor Donor Donor Donor
NK cell NK cell NK cell NK cell
Inhibitory Inhibitory
Activating KIR2DL1 Activating KIR2DL1 Activating Inhibitory Activating Inhibitory
receptor (group 2 receptor (group 2 receptor receptor receptor receptor
specific) specific) Anti-KIR
antibody
Activating HLA-Cw4 Activating HLA-Cw3 Activating HLA-C Activating
ligand (group 2) ligand (group 1) ligand ligand HLA-C
Host Host Host Host
leukemic leukemic leukemic leukemic
blast blast blast blast
Resistance Susceptibility
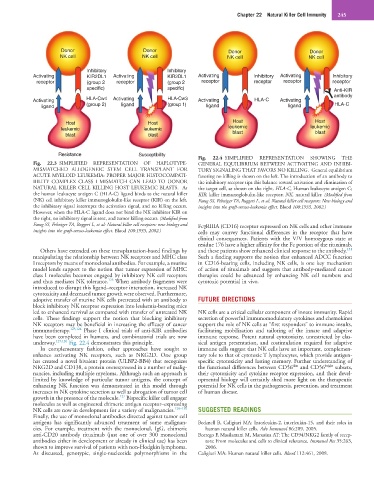
Fig. 22.4 SIMPLIFIED REPRESENTATION SHOWING THE
Fig. 22.3 SIMPLIFIED REPRESENTATION OF HAPLOTYPE- GENERAL EQUILIBRIUM BETWEEN ACTIVATING AND INHIBI-
MISMATCHED ALLOGENEIC STEM CELL TRANSPLANT FOR TORY SIGNALING THAT FAVORS NO KILLING. General equilibrium
ACUTE MYELOID LEUKEMIA: PROPER MAJOR HISTOCOMPATI- favoring no killing is shown on the left. The introduction of an antibody to
BILITY COMPLEX CLASS I MISMATCH CAN LEAD TO DONOR the inhibitory receptor tips this balance toward activation and elimination of
NATURAL KILLER CELL KILLING HOST LEUKEMIC BLASTS. As the target cell, as shown on the right. HLA-C, Human leukocyte antigen C;
the human leukocyte antigen C (HLA-C) ligand binds to the natural killer KIR, killer immunoglobulin-like receptor; NK, natural killer. (Modified from
(NK) cell inhibitory killer immunoglobulin-like receptor (KIR) on the left, Farag SS, Fehniger TA, Ruggeri L, et al: Natural killer cell receptors: New biology and
the inhibitory signal interrupts the activation signal, and no killing occurs. insights into the graft-versus-leukemia effect. Blood 100:1935, 2002.)
However, when the HLA-C ligand does not bind the NK inhibitor KIR on
the right, no inhibitory signal is sent, and tumor killing occurs. (Modified from
Farag SS, Fehniger TA, Ruggeri L, et al: Natural killer cell receptors: new biology and FcγRIIIA (CD16) receptor expressed on NK cells and other immune
insights into the graft-versus-leukemia effect. Blood 100:1935, 2002.) cells may convey functional differences in the receptor that have
clinical consequences. Patients with the V/V homozygous state at
residue 176 have a higher affinity for the Fc portion of the rituximab,
131
Others have extended on these transplantation-based findings by and these patients show enhanced clinical response to the antibody.
manipulating the relationship between NK receptors and MHC class Such a finding supports the notion that enhanced ADCC function
I receptors by means of monoclonal antibodies. For example, a murine in CD16-bearing cells, including NK cells, is one key mechanism
model lends support to the notion that tumor expression of MHC of action of rituximab and suggests that antibody-mediated cancer
class I molecules becomes engaged by inhibitory NK cell receptors therapies could be advanced by enhancing NK cell numbers and
123
and thus mediates NK tolerance. When antibody fragments were cytotoxic potential in vivo.
introduced to disrupt this ligand–receptor interaction, increased NK
cytotoxicity and decreased tumor growth were observed. Furthermore,
adoptive transfer of murine NK cells pretreated with an antibody to FUTURE DIRECTIONS
block inhibitory NK receptor expression into leukemia-bearing mice
led to enhanced survival as compared with transfer of untreated NK NK cells are a critical cellular component of innate immunity. Rapid
cells. These findings support the notion that blocking inhibitory secretion of powerful immunomodulatory cytokines and chemokines
NK receptors may be beneficial in increasing the efficacy of cancer support the role of NK cells as “first responders” to immune insults,
immunotherapy. 123,124 Phase I clinical trials of anti-KIR antibodies facilitating mobilization and tailoring of the innate and adaptive
have been completed in humans, and combinatorial trials are now immune response. Potent natural cytotoxicity, unrestricted by clas-
underway. 125,126 Fig. 22.4 demonstrates this principle. sical antigen presentation, and costimulation required for adaptive
In complementary fashion, other approaches have sought to immune cells suggest that NK cells have an important, complemen-
enhance activating NK receptors, such as NKG2D. One group tary role to that of cytotoxic T lymphocytes, which provide antigen-
has created a novel bivalent protein (ULBP2-BB4) that recognizes specific cytotoxicity and lasting memory. Further understanding of
dim
NKG2D and CD138, a protein overexpressed in a number of malig- the functional differences between CD56 and CD56 bright subsets,
nancies, including multiple myeloma. Although such an approach is their cytotoxicity and cytokine receptor expression, and their devel-
limited by knowledge of particular tumor antigens, the concept of opmental biology will certainly shed more light on the therapeutic
enhancing NK function was demonstrated in this model through potential for NK cells in the pathogenesis, prevention, and treatment
increases in NK cytokine secretion as well as abrogation of tumor cell of human disease.
127
growth in the presence of the molecule. Bispecific killer cell engager
molecules as well as engineered chimeric antigen receptor–expressing
NK cells are now in development for a variety of malignancies. 128–130 SUGGESTED READINGS
Finally, the use of monoclonal antibodies directed against tumor cell
antigens has significantly advanced treatment of some malignan- Becknell B, Caligiuri MA: Interleukin-2, interleukin-15, and their roles in
cies. For example, treatment with the monoclonal, IgG, chimeric human natural killer cells. Adv Immunol 86:209, 2005.
anti-CD20 antibody rituximab (just one of over 300 monoclonal Borrego F, Masilamani M, Marusina AT: The CD94/NKG2 family of recep-
antibodies either in development or already in clinical use) has been tors: From molecules and cells to clinical relevance. Immunol Res 35:263,
shown to improve survival of patients with non-Hodgkin lymphoma. 2006.
As discussed, genotypic, single-nucleotide polymorphisms in the Caligiuri MA: Human natural killer cells. Blood 112:461, 2008.